Question: Question 8 part 1 part 2 part 3 A student is given the question: What is the mass of a gold bar that is 7.279104m3
Question 8

part 1
part 2

part 3
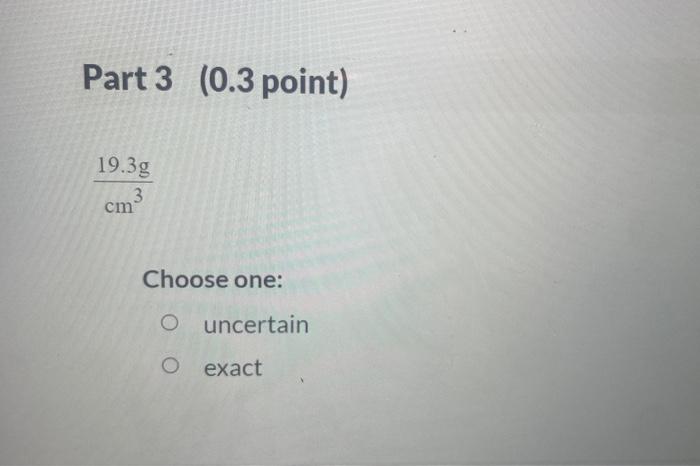
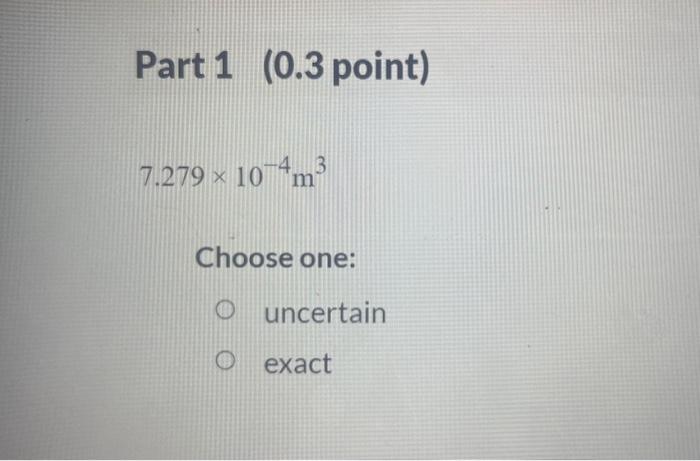
A student is given the question: "What is the mass of a gold bar that is 7.279104m3 in volume? The density of gold is 19.3g/cm ? Thestudent obtains the answer through the following calculation: answsr7.279104m3(1m100cm)3cm319.38 For the above calculation, identify each value as one that is uncertain or known exactly. Part 1 ( 0.3 point) 7.279104m3 Choose one: uncertain exact Part 2 (0.3 point) (1m100cm)3 Choose one: uncertain exact cm319.3g Choose one: uncertain exact
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


