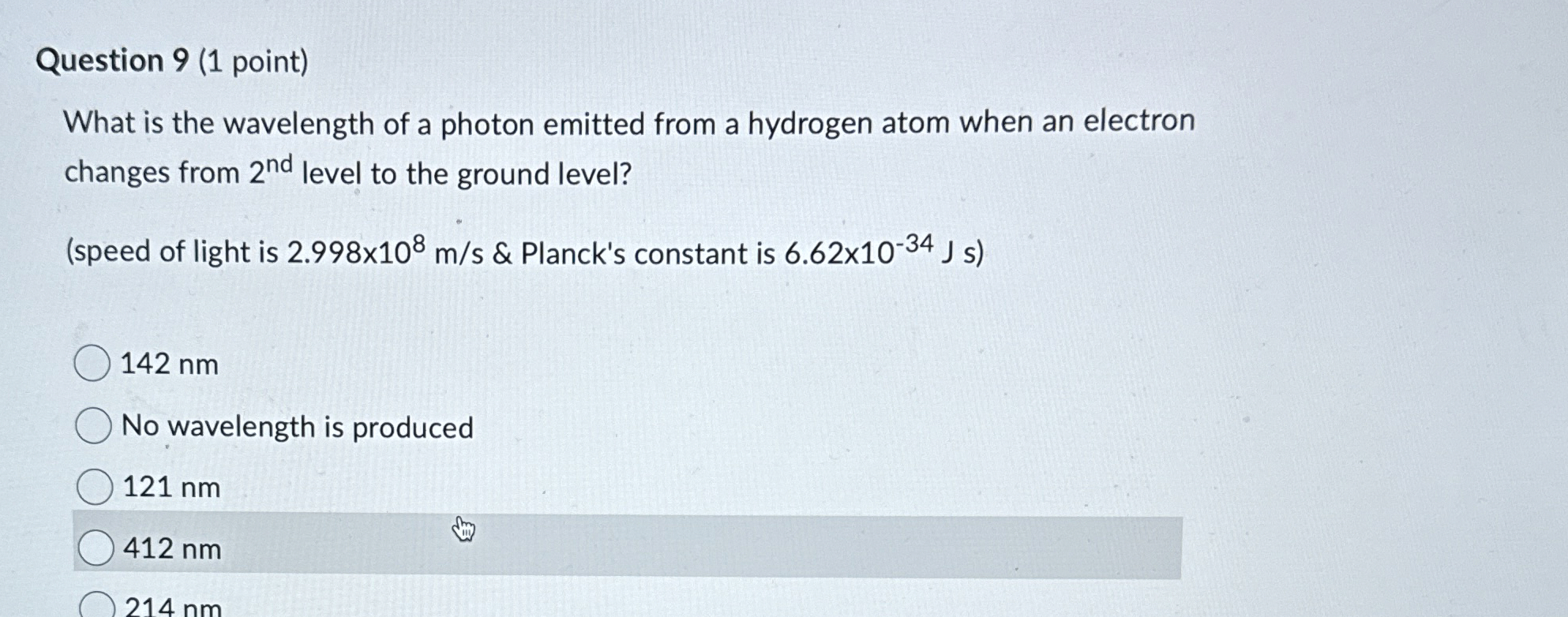
Question: Question 9 ( 1 point ) What is the wavelength of a photon emitted from a hydrogen atom when an electron changes from 2 n
Question point
What is the wavelength of a photon emitted from a hydrogen atom when an electron changes from level to the ground level?
speed of light is & Planck's constant is
nm
No wavelength is produced
nm
nm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


