Question: Question 9 8 pts The following initial rate data were obtained at 25C for the indicated reaction. Determine the rate law for the reaction and
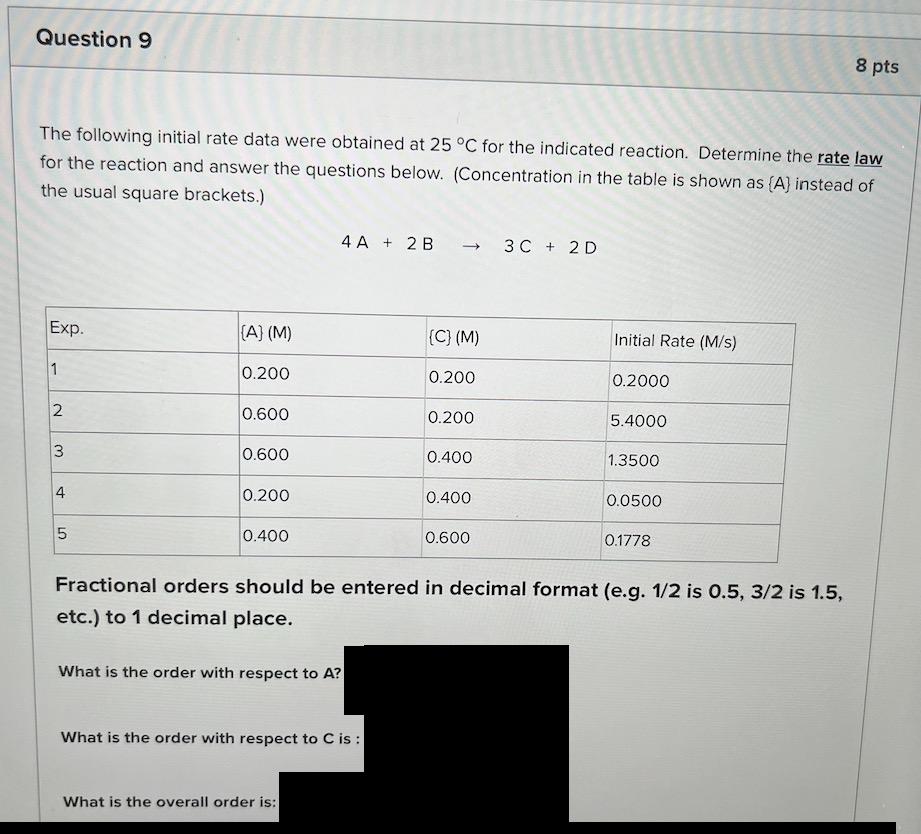
Question 9 8 pts The following initial rate data were obtained at 25C for the indicated reaction. Determine the rate law for the reaction and answer the questions below. (Concentration in the table is shown as (A) instead of the usual square brackets.) 4A + 2B 3 C + 2D -> Exp. (A) (M) (C) (M) Initial Rate (M/s) 1 0.200 0.200 0.2000 N 0.600 0.200 5.4000 3 0.600 0.400 1.3500 4 0.200 0.400 0.0500 5 0.400 0.600 0.1778 Fractional orders should be entered in decimal format (e.g. 1/2 is 0.5, 3/2 is 1.5, etc.) to 1 decimal place. What is the order with respect to A? What is the order with respect to C is: What is the overall order is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


