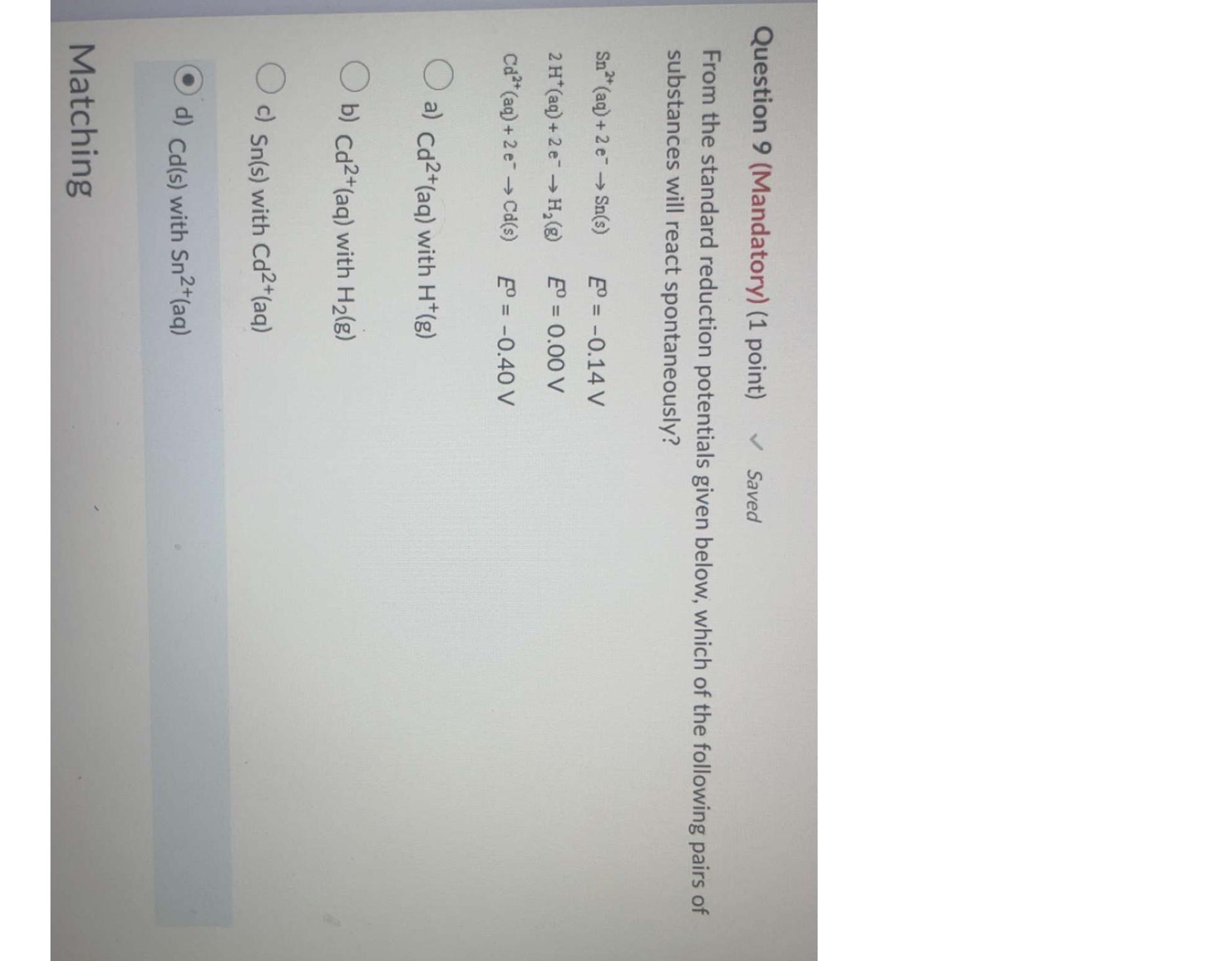
Question: Question 9 ( Mandatory ) ( 1 point ) Saved From the standard reduction potentials given below, which of the following pairs of substances will
Question Mandatory point
Saved
From the standard reduction potentials given below, which of the following pairs of substances will react spontaneously?
a with
b with
c with
d with
Matching
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


