Question: QUESTION: At pH=10, what would be the approximate net charge of the following peptide: ARTQMHLA ANSWER: 0 Could someone please help me determine how this
QUESTION: At pH=10, what would be the approximate net charge of the following peptide: ARTQMHLA
ANSWER: 0
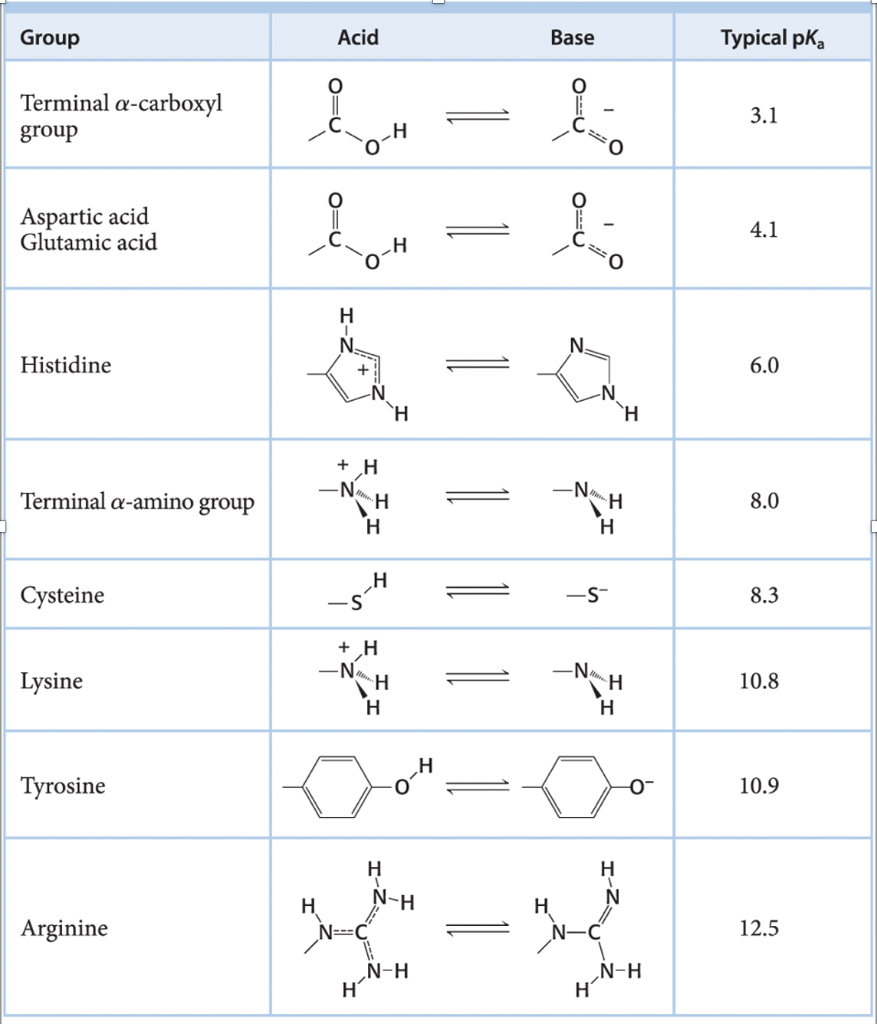
Could someone please help me determine how this net charge was calculated? Below I have included a table of pKa values that we are supposed to utilize for this question. THANK YOU!
S
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


