Question: question data FOR FULL CREDIT, SHOW DETAILED CALCULATION SETUPS. REMEMBER TO FOLLOW THE SIGNIFICANT FIGURES CONVENTION, AND TO SHOW MEASUREMENT UNITS FOR EACH QUANTITY. 1.
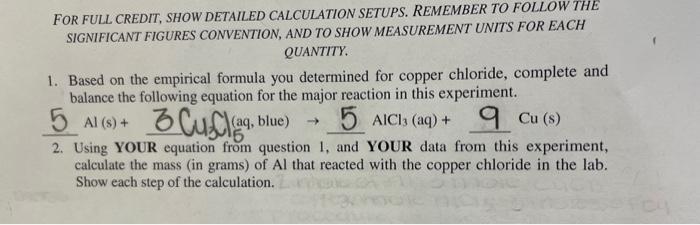


FOR FULL CREDIT, SHOW DETAILED CALCULATION SETUPS. REMEMBER TO FOLLOW THE SIGNIFICANT FIGURES CONVENTION, AND TO SHOW MEASUREMENT UNITS FOR EACH QUANTITY. 1. Based on the empirical formula you determined for copper chloride, complete and balance the following equation for the major reaction in this experiment. 5Al(s)+3Buff(aq,blue)5AlCl3(aq)+Cu(s) 2. Using YOUR equation from question 1, and YOUR data from this experiment, calculate the mass (in grams) of Al that reacted with the copper chloride in the lab. Show each step of the calculation. Mass of copper recovered (show calculation) Mevaporating+Cu-mevaporatingd=(afterlast53.114352.6007g5.136101neatina)=0.51360.5136gCuMassofchlorideinyoursample(showcalculation)1.64g0.51369=1.13g=1.12641.13gClAmount(inmol)ofCuinyoursample(showcalculation)63.55g0.513logCu,63.55gmolCu=8.0821020.008082molCuExperimentalmol/molratio(showcalculation)0.008082/0.008082133 WHETHER RECORDING DATA OR SHOWING CALCULATIONS, REMEMBER TO INCLUDE MEASUREMENT UNITS WITH EACH NUMERICAL VALUE. Volume of copper chloride solution in your sample final buret reading (read to 0.01-mL place) 34.27mL initial buret reading 0.84mL volume of copper chloride solution delivered 24.43mL Mass of copper chloride per mL (from stock solution label) 0.0672g/mL Mass of copper chloride in your sample (show calculation) 1.042>1.64 24.43mL1mol0.0672g/mL Mass of clean, dry, empty evaporating dish 52.6007.g Mass of evaporating dish + copper: after first heating after second heating 53.114353.1159}1+notm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


