Question: Question Format: Short H H O o Formic acid, as shown in the model above, occurs naturally. It is found in some ants and is

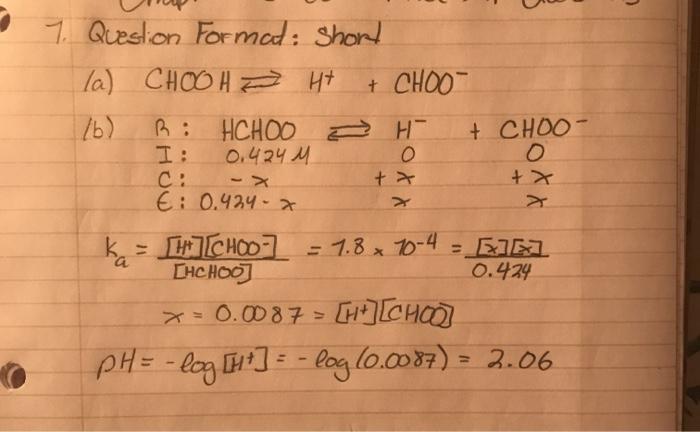
Question Format: Short H H O o Formic acid, as shown in the model above, occurs naturally. It is found in some ants and is injected into the victims of the ant's bite. Its name comes from the Latin words for the fies (Formicidae) and genus (Formica) for ants. It was first isolated by the destructive distilles of ant bodies. Formic acid is a weak acid with a K, value of 1.8 X 10 at 25C. A solution with a concentration of 0.424 M formic acid is prepared in the laboratory. (a) Write the ionization equation for an aqueous solution of formic acid. (b) Determine the pH of the solution prepared above. (c) What is the hybridization of the carbon atom in the formic acid? (d) A buffer solution of formic acid is to be made. Draw the conjugate base (either Low structure or a model as shown above) needed to create the buffer and indicate the loc of any charge. 7. Question Format: short la) CHOOH Ht + CHOO 16) B : HCHOO DH I: 0.424 M + CHOO xxo 4x > E: 0.424 x ka [H]] = [CHOO = 1.8 x 70-4 = EE LHCHOO) 0.424 x = 0.0087 = [+] [chool pH = -log [H+] = -log(0.0087) - 3.06
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


