Question: Question must be solved with Mathematica code. 3) Redlich-Kwong equation of state (first improved version of van der Waals equation is) the relationship between pressure
Question must be solved with Mathematica code.
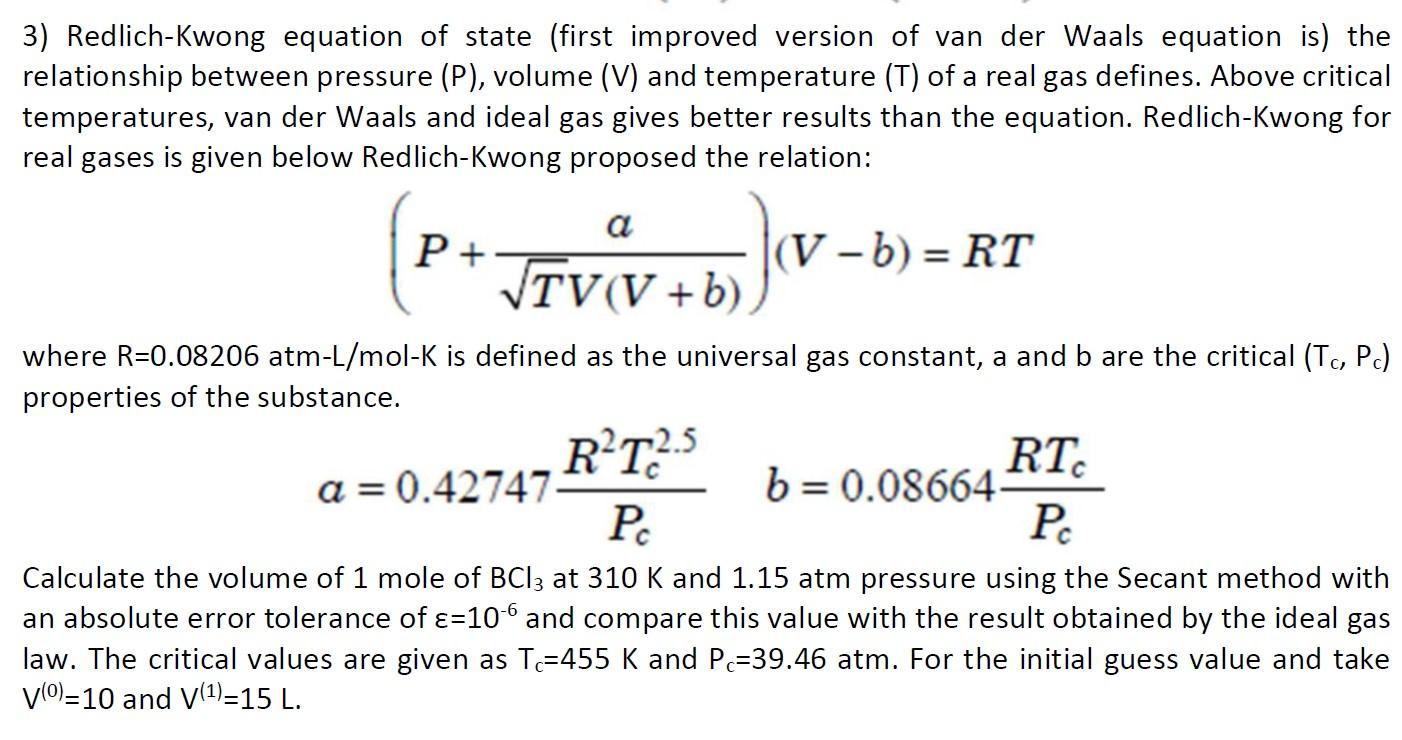
3) Redlich-Kwong equation of state (first improved version of van der Waals equation is) the relationship between pressure (P), volume (V) and temperature (T) of a real gas defines. Above critical temperatures, van der Waals and ideal gas gives better results than the equation. Redlich-Kwong for real gases is given below Redlich-Kwong proposed the relation: (P+TV(V+b)a)(Vb)=RT where R=0.08206atmL/molK is defined as the universal gas constant, a and b are the critical (Tc,Pc) properties of the substance. a=0.42747PcR2Tc2.5b=0.08664PcRTc Calculate the volume of 1 mole of BCl3 at 310K and 1.15 atm pressure using the Secant method with an absolute error tolerance of =106 and compare this value with the result obtained by the ideal gas law. The critical values are given as Tc=455K and Pc=39.46 atm. For the initial guess value and take V(0)=10 and V(1)=15L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


