Question: Question No. 1 ( 20 points): A. Explain what mint by the q-line; show how the q-line equation changes with the feed condition. B. A
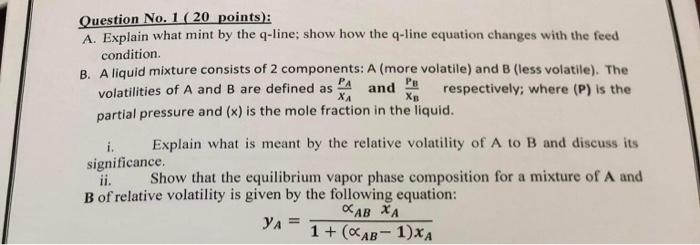
Question No. 1 ( 20 points): A. Explain what mint by the q-line; show how the q-line equation changes with the feed condition. B. A liquid mixture consists of 2 components: A (more volatile) and B (less volatile). The volatilities of A and B are defined as XAPA and XBPB respectively; where (P) is the partial pressure and (x) is the mole fraction in the liquid. i. Explain what is meant by the relative volatility of A to B and discuss its significance. ii. Show that the equilibrium vapor phase composition for a mixture of A and B of relative volatility is given by the following equation: yA=1+(AB1)xAABxA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


