Question: QUESTION THREE ( a ) What is diffusion? ( b ) Why is the diffusion rate in solids slower than that in liquids and gasses?
QUESTION THREE
a What is diffusion?
b Why is the diffusion rate in solids slower than that in liquids and gasses?
c State two mechanisms of diffusion in solids.
table marks marks
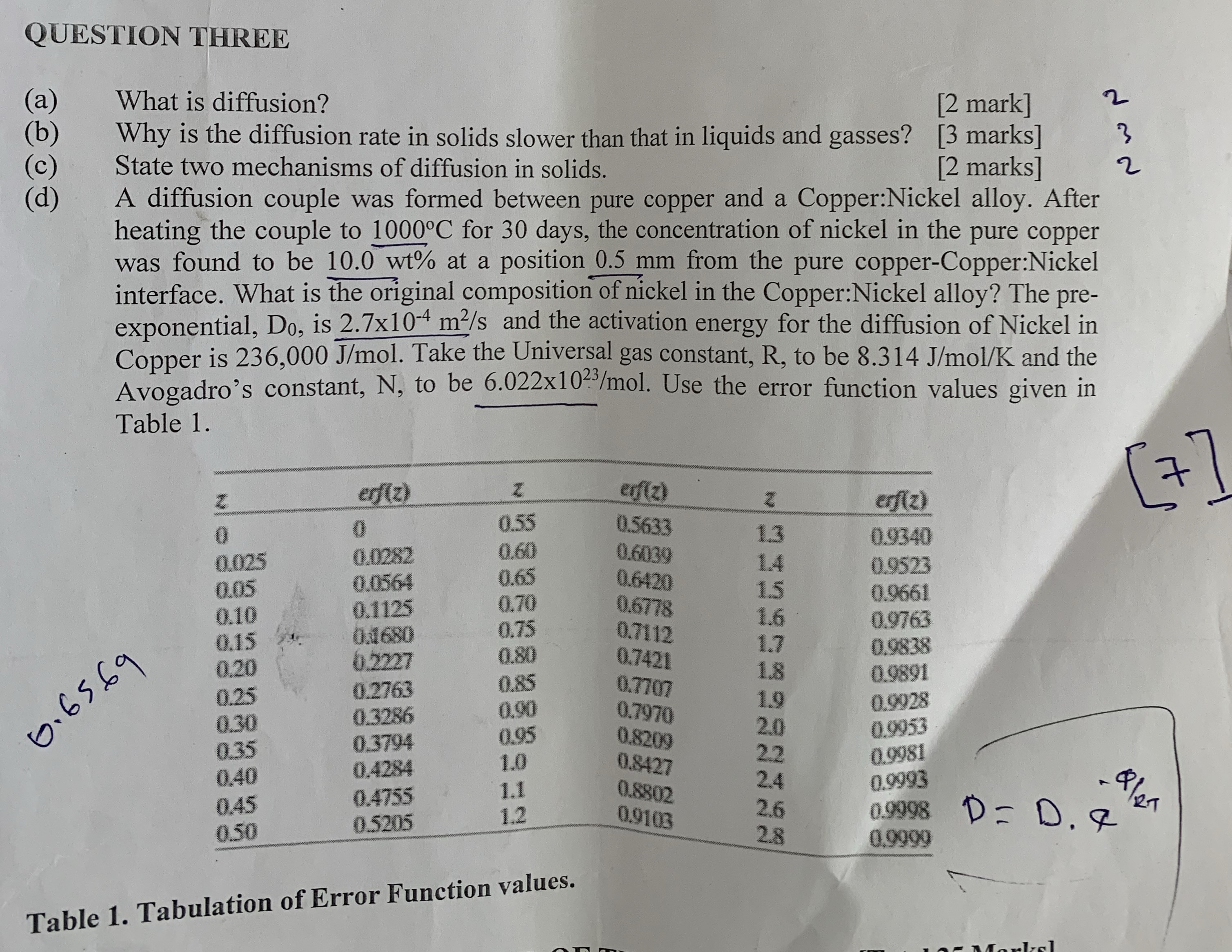
d A diffusion couple was formed between pure copper and a Copper:Nickel alloy. After heating the couple to for days, the concentration of nickel in the pure copper was found to be at a position mm from the pure copperCopper:Nickel interface. What is the original composition of nickel in the Copper:Nickel alloy? The preexponential, is and the activation energy for the diffusion of Nickel in Copper is Take the Universal gas constant, R to be and the Avogadro's constant, N to be Use the error function values given in Table
tableefzeffzeffz
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


