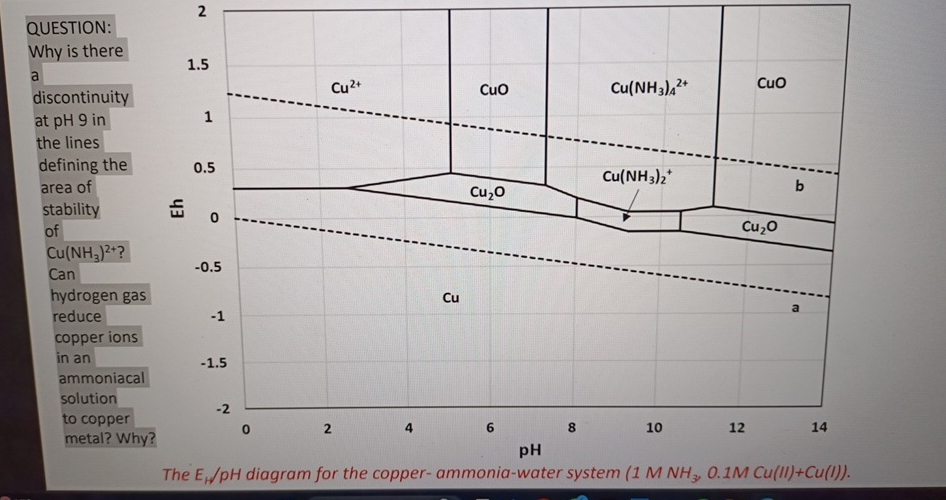
Question: QUESTION: Why is there a discontinuity at p H 9 in the lines defining the area of stability of C u ( N H 3
QUESTION:
Why is there
a
discontinuity
at in
the lines
defining the
area of
stability of
Can
hydrogen gas
reduce
copper ions
in an
ammoniacal
solution
to copper
metal? Why?
The diagram for the copperammoniawater system :MCU
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


