Question: Question1 Question2 Question3 A solution was prepared by dissolving 38.0 gg of KClKCl in 225 gg of water. NaCl is relatively soluble in water, yet
Question1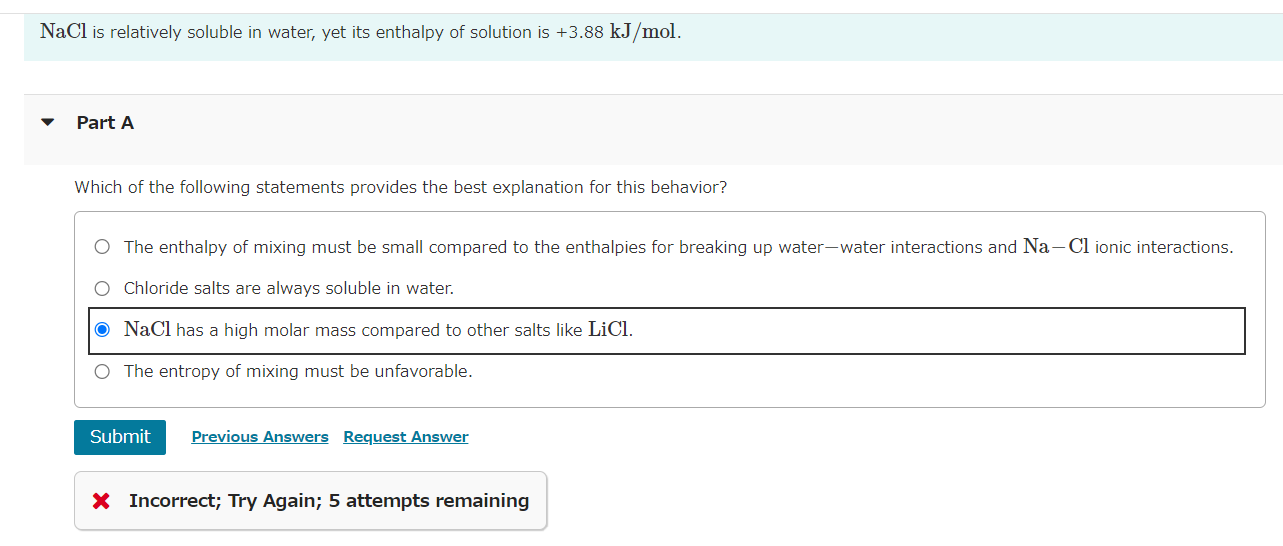
Question2
Question3
A solution was prepared by dissolving 38.0 gg of KClKCl in 225 gg of water.
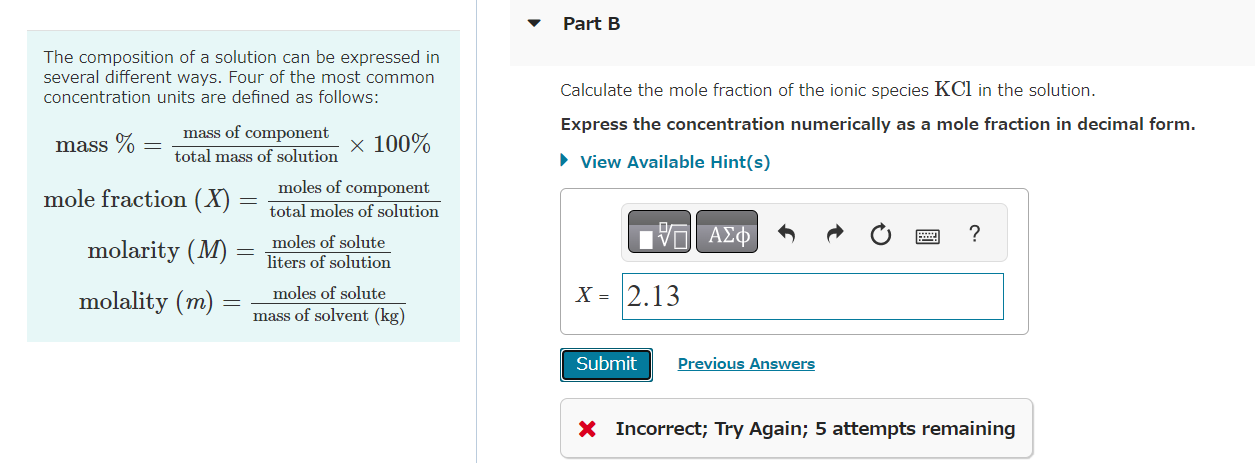
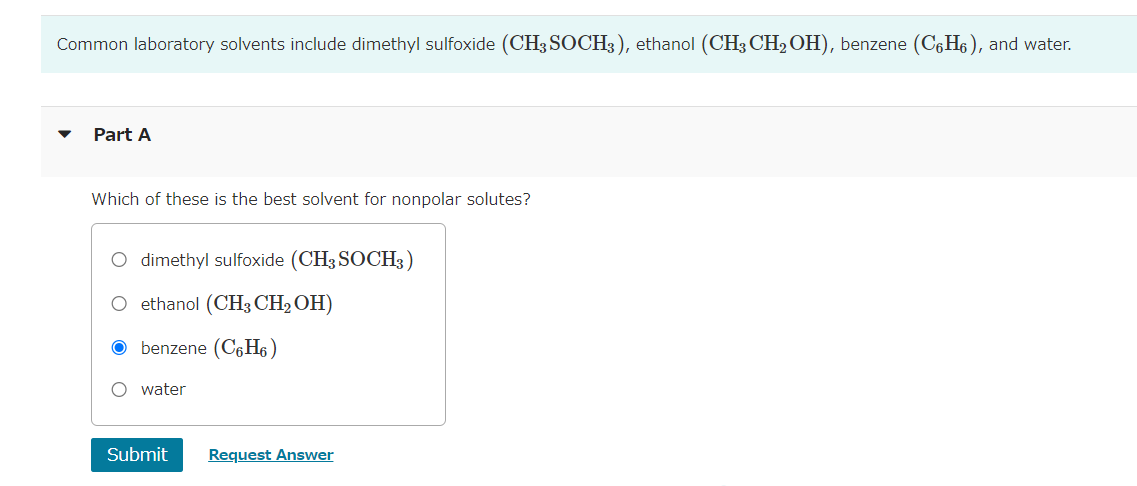
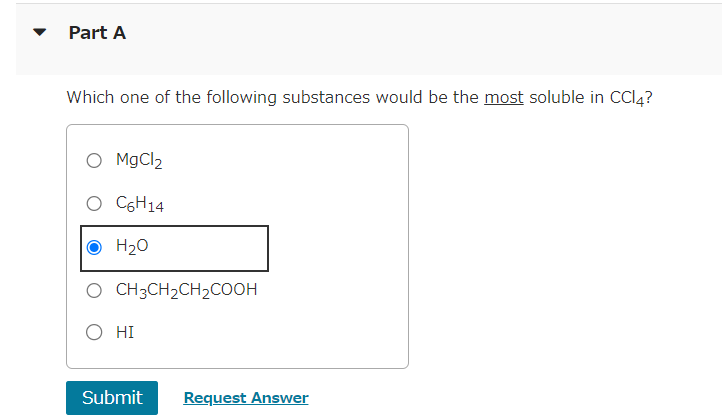
NaCl is relatively soluble in water, yet its enthalpy of solution is +3.88kJ/mol. Part A Which of the following statements provides the best explanation for this behavior? The enthalpy of mixing must be small compared to the enthalpies for breaking up water-water interactions and NaCl ionic interactions. Chloride salts are always soluble in water. NaCl has a high molar mass compared to other salts like LiCl. The entropy of mixing must be unfavorable. x Incorrect; Try Again; 5 attempts remaining Common laboratory solvents include dimethyl sulfoxide (CH3SOCH3), ethanol (CH3CH2OH), benzene (C6H6), and water. Part A Which of these is the best solvent for nonpolar solutes? dimethyl sulfoxide (CH3SOCH3) ethanol (CH3CH2OH) benzene (C6H6) water Which of the following solvents will most effectively dissolve paraffin wax, which is a mixture of large hydrocarbon molecules (alkanes) containing between 20 and 40 carbon atoms? hexane (C6H14) acetonitrile (CH3CN) water (H2O) acetone (CH3COCH3) Part A Which one of the following substances would be the most soluble in CCl4 ? MgCl2 C6H14 H2O CH3CH2CH2COOH HI Submit Request Answer The composition of a solution can be expressed in several different ways. Four of the most common concentration units are defined as follows: Calculate the mole fraction of the ionic species KCl in the solution. mass%=totalmassofsolutionmassofcomponent100%molefraction(X)=totalmolesofsolutionmolesofcomponentmolarity(M)=litersofsolutionmolesofsolutemolality(m)=massofsolvent(kg)molesofsolute Express the concentration numerically as a mole fraction in decimal form
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


