Question: Quiz: Module 4: Midterm (quiz x Module 1: Exercises - Practice | x Module 2: Quiz (required, grad x Module 3: Quiz (required, grad x
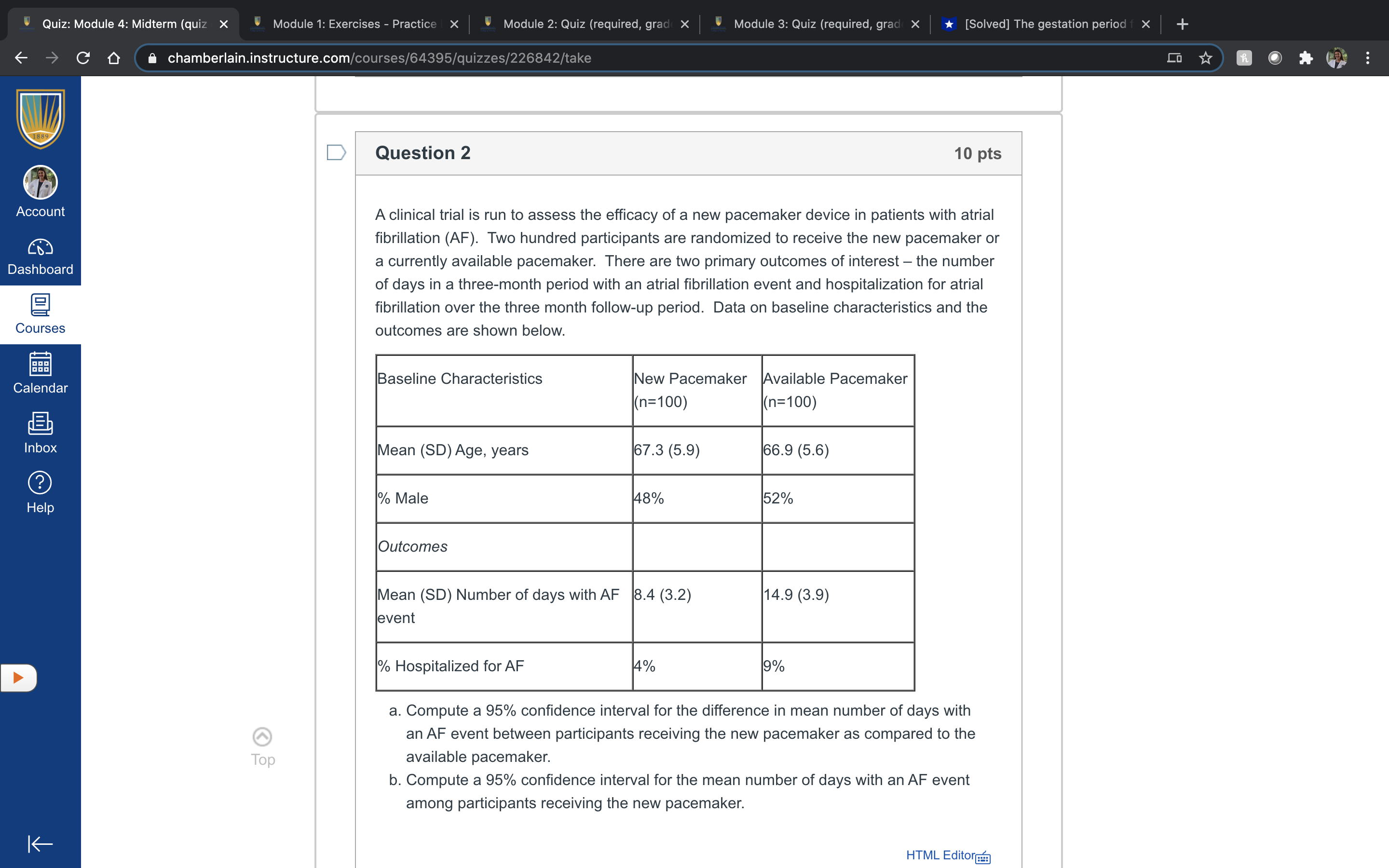
Quiz: Module 4: Midterm (quiz x Module 1: Exercises - Practice | x Module 2: Quiz (required, grad x Module 3: Quiz (required, grad x [Solved] The gestation period f X CO chamberlain.instructure.com/courses/64395/quizzes/226842/take Co D Question 2 10 pts Account A clinical trial is run to assess the efficacy of a new pacemaker device in patients with atrial fibrillation (AF). Two hundred participants are randomized to receive the new pacemaker or Dashboard a currently available pacemaker. There are two primary outcomes of interest - the number of days in a three-month period with an atrial fibrillation event and hospitalization for atrial fibrillation over the three month follow-up period. Data on baseline characteristics and the Courses outcomes are shown below. Calendar Baseline Characteristics New Pacemaker Available Pacemaker (n=100) (n=100) Inbox Mean (SD) Age, years 67.3 (5.9) 66.9 (5.6) ? Help % Male 48% 52% Outcomes Mean (SD) Number of days with AF 8.4 (3.2) 14.9 ( 3.9 ) event % Hospitalized for AF 4% 9% a. Compute a 95% confidence interval for the difference in mean number of days with an AF event between participants receiving the new pacemaker as compared to the Top available pacemaker. b. Compute a 95% confidence interval for the mean number of days with an AF event among participants receiving the new pacemaker. K HTML Editor (#29
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


