Question: | Reaction Engineering | please solve and explain question properly. do not copy paste A P+s, which is a first order reaction with respect to
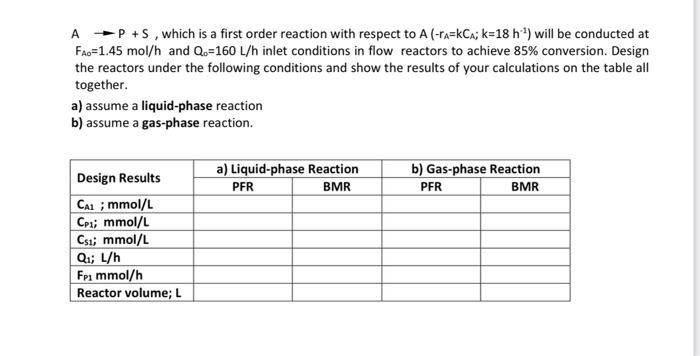
A P+s, which is a first order reaction with respect to A (-ta=kCa; k=18 h) will be conducted at Fao=1.45 mol/h and Q.=160 L/h inlet conditions in flow reactors to achieve 85% conversion. Design the reactors under the following conditions and show the results of your calculations on the table all together. a) assume a liquid-phase reaction b) assume a gas-phase reaction. a) Liquid-phase Reaction PER BMR b) Gas-phase Reaction PFR BMR Design Results Cal; mmol/L Cpii mmol/L Csi; mmol/L Qi; L/h Fpi mmol/h Reactor volume; L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


