Question: Reaction Rate Exercise 6. The rate equation for 2A + B 2C + 3D is: d[C] K[A][B][C] dt Express the rate equation as a function
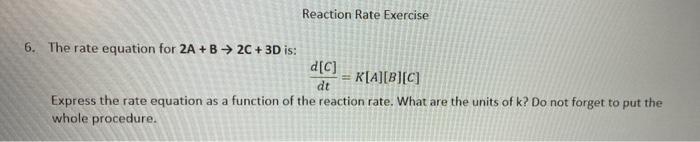
Reaction Rate Exercise 6. The rate equation for 2A + B 2C + 3D is: d[C] K[A][B][C] dt Express the rate equation as a function of the reaction rate. What are the units of k? Do not forget to put the whole procedure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


