Question: Read the Merck Vioxx case. - Why do you think it took Merck 5 years to remove Vioxx from the market? - What aspects of
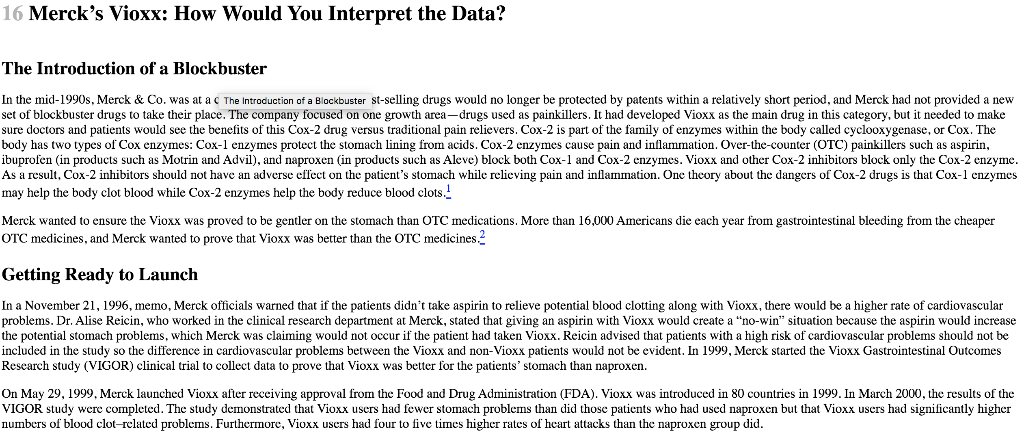





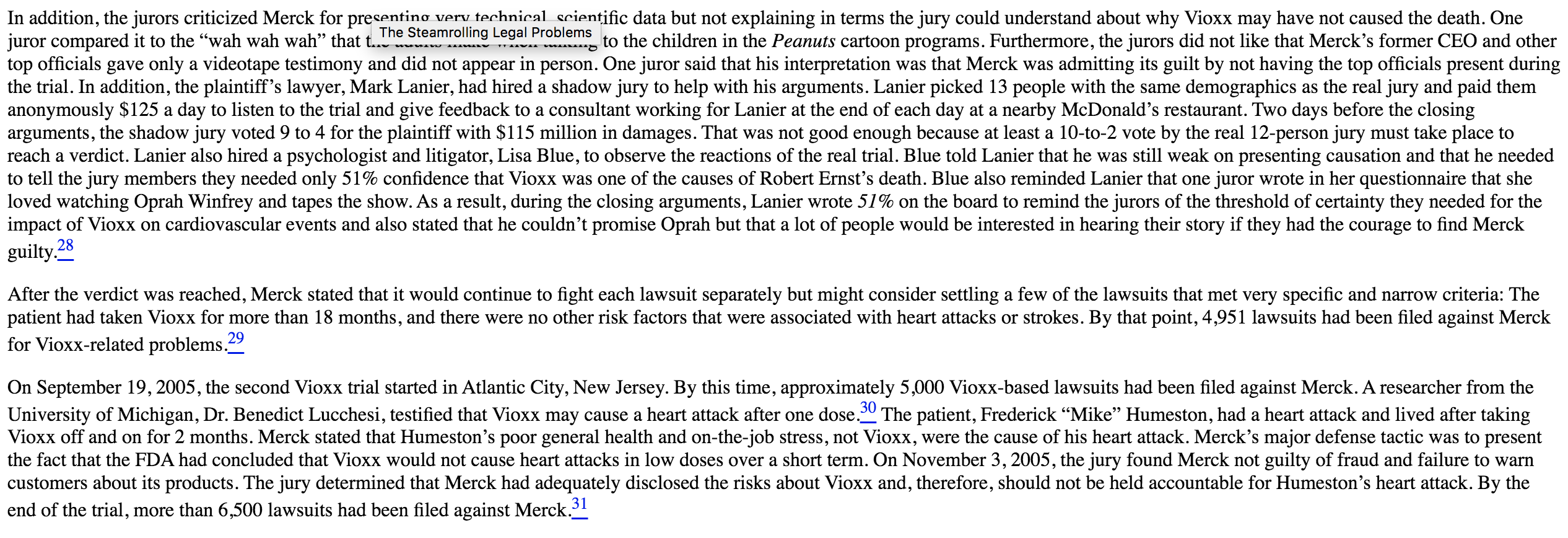




Read the Merck Vioxx case. - Why do you think it took Merck 5 years to remove Vioxx from the market? - What aspects of Merck's culture needed change? - How likely is it that change has been accomplished in your opinion? The Introduction of a Blockbuster In the mid-1990s, Merck \& Co. was at a c The introduction of a Blockbuster st-selling drugs would no longer be protected by patents within a relatively short period, and Merck had not provided a new set of blockbuster drugs to take their place. The company focused on one growth area-drugs used as painkillers. It had developed Vioxx as the main drug in this category, but it needed to make sure doctors and patients would see the benefits of this Cox-2 drug versus traditional pain relievers. Cox-2 is part of the family of enzymes within the body called cyclooxygenase, or Cox. The body has two types of Cox enzymes: Cox-1 enzymes protect the stomach lining from acids. Cox-2 enzymes cause pain and inflammation. Over-the-counter (OTC) painkillers such as aspirin, ibuprofen (in products such as Motrin and Advil), and naproxen (in products such as Aleve) block both Cox-1 and Cox-2 enzymes. Vioxx and other Cox-2 inhibitors block only the Cox-2 enzyme. As a result, Cox-2 inhibitors should not have an adverse effect on the patient's stomach while relieving pain and inflammation. One theory about the dangers of Cox-2 drugs is that Cox-1 enzymes may help the body clot blood while Cox-2 enzymes help the body reduce blood clots.. Merck wanted to ensure the Vioxx was proved to be gentler on the stomach than OTC medications. More than 16,000 Americans die each year from gastrointestinal bleeding from the cheaper OTC medicines, and Merck wanted to prove that Vioxx was better than the OTC medicines.' Getting Ready to Launch In a November 21, 1996, memo, Merck officials warned that if the patients didn't take aspirin to relieve potential blood clotting along with Vioxx, there would be a higher rate of cardiovascular problems. Dr. Alise Reicin, who worked in the clinical research department at Merck, stated that giving an aspirin with Vioxx would create a "no-win" situation because the aspirin would increase the potential stomach problems, which Merck was claiming would not occur if the patient had taken Vioxx. Reicin advised that patients with a high risk of cardiovascular problems should not be included in the study so the difference in cardiovascular problems between the Vioxx and non-Vioxx patients would not be evident. In 1999 , Merck started the Vioxx Gastrointestinal Outcomes Research study (VIGOR) clinical trial to collect data to prove that Vioxx was better for the patients' stomach than naproxen. On May 29, 1999, Merck launched Vioxx after receiving approval from the Food and Drug Administration (FDA). Vioxx was introduced in 80 countries in 1999. In March 2000, the results of the VIGOR study were completed. The study demonstrated that Vioxx users had fewer stomach problems than did those patients who had used naproxen but that Viox users had significantly higher numbers of blood clot-related problems. Furthermore, Vioxx users had four to five times higher rates of heart attacks than the naproxen group did. On March 9, 2000, Merck's research head, Edward Scolnick, emailed his colleagues at Merck and warned them, based on the results of the VIGOR study, that Vioxx created a higher risk of a cardiovascular event. He called the side effects a shame but stated that there are always hazards with using drugs. Scolnick also asked that additional data be developed to show that this result occurred in all Cox-2 inhibitors, not just Vioxx. Merck sent out a press release describing the results of the VIGOR study. In contrast to the concerns raised by Scolnick and others at Merck, the press release was titled "Merck Confirms Favorable Cardiovascular Safety Profile of Vioxx." In the press release, Merck did acknowledge the results of the study but also highlighted other clinical trials and other data collected that had shown "no difference" in the rates of cardiovascular events for patients using Vioxx and a placebo or OTC pain relievers. Merck explained that the antiplatelet properties of naproxen might have helped the patients who took it. 3 In November 2000, the New England Journal of Medicine published the results of a VIGOR study. The article was written by academic researchers who had received research grants or consulting contracts from Merck. The paper touted the potential benefits of Vioxx for stomach problems and heart attacks. The authors did not provide any information about the higher risk of serious cardiovascular complications from using Vioxx, such as strokes and blood clots. Based on the data in the study, the authors had concluded that Vioxx did not show a significant rise in heart attacks among patients who were not at high risk for heart attacks before they started taking Vioxx.- This study demonstrated that Vioxx patients were four times more likely to have a heart attack or stroke (0.4% to 0.1%) than were those patients who took the OTC drug naproxen. However, the focus on the paper was Vioxx's benefits. Merck argued that the results of the study could have beer based on the protective effects of naproxen, rather than on the dangers of Vioxx. 5 In February 2001, Merck won a significant decision from the FDA when the FDA allowed Merck to claim that Vioxx is safer for patients' stomachs than previous painkillers, giving Vioxx a significant competitive advantage over its chief rival, Celebrex, made by Pfizer. The FDA wanted to have the dangers of cardiovascular problems displayed prominently on the Vioxx label, but Merck refused, arguing that it wanted to promote the positive aspects of the drug. They compromised, resulting in a label showing the positive impact on stomach problems first, then the information about the risks of heart attacks and strokes. The new labeling was released in April 2002. In August 2001, the results of a Vioxx study done by the Cleveland Clinic were published in the Journal of the American Medical Association. Researchers concluded that a cautionary flag needed o be raised concerning the increased risk of cardiovascular events occurring in patients who use Vioxx. The authors stated that all Cox-2 inhibitors may increase the risk, but Vioxx appeared to be tiskier than the other drugs in the marketplace. Merck met with the authors to argue that Merck did not believe that there was a problem with the drug and asked the journal to allow Merck to oresent a rebuttal to their findings. The journal refused Merck's request.. On September 17, 2001, the FDA sent a letter to Merck's CEO, Raymond Gilmartin, warning Merck that it should stop marketing Vioxx as a drug with a minimal threat of cardiovascular oroblems when comparing Vioxx with naproxen. The FDA stated explicitly that Merck's promotional campaign discounted the fact that patients who had used Vioxx were observed to have four to live times higher risk of a heart attack than those patients in the other sample, which it claimed was a critical fact. The FDA also criticized Merck for claiming that the reason Vioxx patients had more heart attacks was because of the protective properties that were found in naproxen. The FDA claimed that Merck had no proof that naproxen had any protective properties for the patients? hearts. In addition, the FDA claimed that Merck was misleading the public by titling the press release of the VIGOR study "Merck Confirms Favorable Cardiovascular Safety Profile of Vioxx." - How to Address the Concerns An internal Merck marketing document for sales representatives told the reps to "dodge" any potentially tough questions that were asked by doctors about the side effects of Vioxx. This advice was part of the "obstacle handling guide" given to help the sales reps continue to convince doctors to prescribe Vioxx. When a doctor asked specifically about concerns related to heart problems, he sales rep was instructed to tell the doctor that Vioxx would not reduce the level of cardiovascular problems and that Vioxx would not be considered a substitute for aspirin. The sales reps were given a 16-page "Dodge Ball Vioxx" document that went through a step-by-step process teaching reps how to respond to questions from doctors about Vioxx. The final four pages of the document had the single word DODGE on each page. How to Address the Concerns In addition, Merck attempted to control academics who started to question the benefits of Vioxx. Stanford University's Dr. Gurkipal Singh was a leading expert in Cox-2 research and was oresenting lectures that were funded by Merck and other companies. Singh asked Merck for additional information pertaining to cardiovascular issues, and Merck refused to provide any. Singh added a slide to his PowerPoint presentations-a picture of a man hiding underneath a blanket-to represent the missing data that Merck would not supply. Merck canceled Singh's subsequent lectures, and the company also complained to Stanford University Medical School that Singh was anti-Merck and anti-Vioxx and was acting irresponsibly. Merck warned Stanford that if Singh's actions continued, there would be consequences for the doctor and for Stanford. Stanford replied that Merck had crossed the line and that other top-tier medical schools had also complained about Merck's threatening behaviors toward rescarchers who had studied Vioxx. Merck CEO Gilmartin responded that Merck had a strong commitment to have strong ethical standards with its dealings with all doctors and health-care providers. The Wonder Drug for Everyone In October 2003, a research study by Merck demonstrated that Vioxx could significantly help children with juvenile rheumatoid arthritis. The results showed that the children in the study were able to tolerate Vioxx, and Merck started moving toward positioning Vioxx as a pediatric medication... However, during the same month, another study funded by Merck showed that patients who had taken Vioxx had a 39% higher risk of heart attack within the first 90 days of taking the medication compared with Celebrex. Despite the results of that study, Merck continued to broaden the scope of uses for Vioxx. On April 1, 2004, Merck announced that the FDA had approved Vioxx to help reduce the pain associated with migraines in adults. Vioxx was the only Cox-2-specific inhibitor the FDA allowed to be prescribed for migraine relief. 11 The Data Continue On April 14, 2004, the results of a clinical trial sponsored by Merck and Harvard Medical School that were to be published in the American Heart Association's publication concluded that Vioxx had higher risks of heart attacks in the first 90 days of use of than Celebrex did. Merck had requested that the authors alter the conclusions so they were not as negative about the side effects of Vioxx. The authors refused to change the conclusion. On May 4, 2004, just hefore the paper was to be published, Merck had one of the researchers, Carolyn Cannuscio, who was an employee at Merck, removed from the article. 12 On August 25, 2004, the results of an FDA study on Vioxx showed increased risk of heart attacks and sudden cardiac deaths. In the study, patients who took doses of Vioxx that were more than 25 milligrams daily had 3.15 times more chance of having a heart problem than did patients in the control group. Although only 10 cases of heart problems were discovered in the study, the results were statistically significant. The conclusion of the study was that Celebrex "may be safer" than Vioxx for heart problems. The results of the study raised concerns at Kaiser Permanente for its customers, and the HMO was considering no longer allowing Vioxx to be included in its drug program. Kaiser helped fund the study with the FDA by giving technical, clinical, and programming support. The spokesperson at Merck, Mary Elizabeth Blake, stated that Merck did not agree with the results and those previous randomized studies, which included thousands of elderly patients, did not find any significant increase in heart problems with the patients. At the time of the results of the study, Vioxx's market share had fallen from 43% in 2001 to 32% in July 2004 . 13 On September 8, 2004, the FDA announced that Vioxx was approved to be used to help children with juvenile rheumatoid arthritis with the only limitation being that the children must be at least 2 ycars old and weigh at least 22 pounds. 14 On September 24, 2004, Merck CEO Gilmartin received a call from the head of Merck's research department, Peter Kim. Kim had been notified the day before that an external panel that was supervising a clinical trial on Vioxx had told Merck to stop the trial immediately because the patients taking Vioxx were twice as likely to have a heart attack or stroke than were those taking a placebo in the control group. The trial lasted for more than 18 months and involved 2,600 patients. There were 15 heart attacks or strokes for every 1,000 patients taking Vioxx versus 7.5 heart attacks or strokes per 1,000 for those patients taking a placebo. There was no difference in the two groups for the first 18 months; both samples had 3.75 heart attacks or strokes per 1,000 patients in the first 18 months. 15 On September 30, 2004, Merck announced that it was withdrawing Vioxx from the market based on studies that linked it to increased occurrences of heart problems. Vioxx had global sales in 2003 of $2.5 billion and more than 100 million prescriptions for the drug had been written since 1999 , when the drug was introduced into the marketplace. Vioxx had accounted for 11% of Merck's global sales in 2003 and was estimated to contribute 20% of Merck's overall level of profitability. Approximately 2 million people had been taking Vioxx when the withdrawal was announced. 16 Sales of Vioxx had dropped in the second quarter of 2004 to $653 million from $801 million in the second quarter of 2003 . With the announcement of the withdrawal, critics started asking whether Gilmartin should resign so Merck could regain its creditability. The cost of the cancellation of Vioxx was estimated to be between $700 million and $750 million for patient ceimbursement and the write-off of the Vioxx pills that had already been manufactured. The cost of cancellation did not include any reserves being set aside for the settlements of future lawsuits. 17 Critics of the FDA raised concerns about why it took so long to pull the drug when questions about its impact on heart problems were first discovered in 2000 . In addition, some critics were asking whether Vioxx was worth both the physical and financial cost. One Vioxx pill sold for as much as $2.50, providing almost the same pain relief as aspirin, though Vioxx users were less likely to develop ulcers and stomach problems versus aspirin users. 18 Within a week of the announcement by Merck, it was estimated that Vioxx-based litigation could cost Merck more than \$10 billion with an estimated 60,000 potential lawsuits to be filed against the pharmaceutical company. 19 The Steamrolling Legal Problems On November 8,2004 , the Department of Justice (DOJ) started a criminal investigation pertaining to Merck's handling of the Vioxx issue. In addition, the SEC notified Merck that it would be starting an investigation. Merck responded by stating that it had acted responsibly and appropriately to both the development and marketing of Vioxx. 20 In his speech on cthics at the University of Michigan in November 2004, Gilmartin explained that patients come first at Merck and that Merck's withdrawal of Vioxx was a clear representation of the values that Merck upholds. Gilmartin reminded the students that ethics is all about your behavior and not what people say about you. He also reminded them that all that matters at the end of the day is what you have done. Gilmartin commented that the Wall Street Journal had misrepresented the facts and policies of Merck when it printed that Merck knew in 2000 that there were problems with Vioxx. In February 2005, Merck announced that it would consider reintroducing Vioxx into the marketplace if the FDA advisory committee decided that the risks associated with Vioxx were also found in other Cox-2 drugs. Merck argued that if the benefits of Vioxx outweighed the risks for some patients, that Vioxx could be sold to patients with appropriate warnings. Of course, the reintroduction of Vioxx would help Merck's legal argument that despite the risks, Vioxx is a valid alternative treatment for some patients. 22 Also in February 2005 , Merck announced that Gilmartin would receive a bonus of $1.4 million for his effort in 2004 . Using its own judgment and discretion, the board of directors at Merck determined the amount of Gilmartin's bonus. 23 On May 5, 2005, Gilmartin stepped down as CEO of Merck and was replaced by Richard Clark, who had heen in charge of manufacturing at Merck. It was speculated that Merck wanted a new CEC from outside the company, but reportedly, the potential candidates Merck was interested in did not want the position. Clark was not given the chairman of the board position, which instead was to be composed of a three-person executive board committee to advise Clark. Gilmartin was to retire from Merck in March 2006, and he remain at Merck after he stepped down as CEO as a special advisor to the board's executive committee. By the beginning of Clark's tenure, more than 2,400 lawsuits had been filed against Merck by the families of former Vioxx patients. 24 The first Vioxx-related trial with a jury started on July 14, 2005, in Angleton, Texas. The plaintiff's lawyer called Merck's marketing campaign the ability to turn science into science fiction and referred to the company's "Merck-y ethics." The Vioxx patient had died from arrhythmia, or an irregular heartbeat, and Vioxx had never been associated with causing that heart problem. By the start of this trial, the number of lawsuits against Merck for Vioxx-related problems had risen to 4,000 with the potential of between 20,000 and 100,000 total cases. 25 On August 19,2005 , the jurfound in favor of the plaintiff in the trial against Merck. The jury found Merck guilty of wrongful death and awarded total damages of \$253 million to Carol Ernst, whose husband, Robert, died after taking Vioxx for 8 months. Of the $253 million, \$229 million were punitive damages for Merck's liability, negligence, and malice. The state of Texas has a limit of $2 million for punitive damages in any trial located in that state, so if the award was paid out, it would automatically be reduced by $227 million. Merck said it would appeal the verdict. The stock price of Merck fell almost 8% to just above $28 per share. 26 The jurors said that they were troubled by seeing the internal documents that showed the company appeared to suppress the risk of Vioxx in its marketin. campaigns. The jurors also stated that Merck needed to make the warning clearer on the packaging. They explained that the warnings needed to be in layperson's terms so patients can decide whether it is worth the risk of taking the drug. 27 In addition, the jurors criticized Merck for presentino very technical scientific data but not explaining in terms the jury could understand about why Vioxx may have not caused the death. One juror compared it to the "wah wah wah" that t... See Steamrolling Legal Problems sto the children in the Peanuts cartoon programs. Furthermore, the jurors did not like that Merck's former CEO and other top officials gave only a videotape testimony and did not appear in person. One juror said that his interpretation was that Merck was admitting its guilt by not having the top officials present during the trial. In addition, the plaintiff's lawyer, Mark Lanier, had hired a shadow jury to help with his arguments. Lanier picked 13 people with the same demographics as the real jury and paid them anonymously $125 a day to listen to the trial and give feedback to a consultant working for Lanier at the end of each day at a nearby McDonald's restaurant. Two days before the closing arguments, the shadow jury voted 9 to 4 for the plaintiff with $115 million in damages. That was not good enough because at least a 10 -to-2 vote by the real 12 -person jury must take place to reach a verdict. Lanier also hired a psychologist and litigator, Lisa Blue, to observe the reactions of the real trial. Blue told Lanier that he was still weak on presenting causation and that he needed to tell the jury members they needed only 51% confidence that Vioxx was one of the causes of Robert Ernst's death. Blue also reminded Lanier that one juror wrote in her questionnaire that she loved watching Oprah Winfrey and tapes the show. As a result, during the closing arguments, Lanier wrote 51% on the board to remind the jurors of the threshold of certainty they needed for the impact of Vioxx on cardiovascular events and also stated that he couldn't promise Oprah but that a lot of people would be interested in hearing their story if they had the courage to find Merck guilty. 28 After the verdict was reached, Merck stated that it would continue to fight each lawsuit separately but might consider settling a few of the lawsuits that met very specific and narrow criteria: The patient had taken Vioxx for more than 18 months, and there were no other risk factors that were associated with heart attacks or strokes. By that point, 4,951 lawsuits had been filed against Merck for Vioxx-related problems. On September 19, 2005, the second Vioxx trial started in Atlantic City, New Jersey. By this time, approximately 5,000 Vioxx-based lawsuits had been filed against Merck. A researcher from the University of Michigan, Dr. Benedict Lucchesi, testified that Vioxx may cause a heart attack after one dose. 30 The patient, Frederick "Mike" Humeston, had a heart attack and lived after taking Vioxx off and on for 2 months. Merck stated that Humeston's poor general health and on-the-job stress, not Vioxx, were the cause of his heart attack. Merck's major defense tactic was to present the fact that the FDA had concluded that Vioxx would not cause heart attacks in low doses over a short term. On November 3, 2005, the jury found Merck not guilty of fraud and failure to warn customers about its products. The jury determined that Merck had adequately disclosed the risks about Vioxx and, therefore, should not be held accountable for Humeston's heart attack. By the end of the trial, more than 6,500 lawsuits had been filed against Merck. 31 On November 30,2005 , the third Vioxx trial, the first tried in federal court, started in Houston, Texas. The widow of Richard "Dicky" Irwin sued Merck, claiming that Vioxx was the cause of her husband's fatal heart attack in 2001. On December 8, 2005, while the third Vioxx trial was in progress, the New England Journal of Medicine published an article titled "Expression of Concern" about the results of the Vioxx study that was published in 2000. The article revealed the academics who had received grant or consulting moncy from Merck and had concluded that Vioxx was better for stomach problems but did not increase the risk of heart attacks. The New England Journal of Medicine had discovered that three Vioxx patients in the study who had suffered heart attacks were excluded from the study and were, therefore, not included in the final data results. The exclusion of the three patients made the results appear that Vioxx was much safer than it should have been. Merck responded by stating that the three heart attacks were not included in the study because they occurred after a predetermined cutolI date for data collection for the study. The executive editor of the journal discovered the omissi The Stoamroling Legal Problerms after being deposed by one of the plaintiffs who was suing Merck. 32 After the journal made its announcement, the plaintiffs in the third Merck trial asked for a mistrial because of the new information pertaining to the VIGOR study. 33 On December 12 , 2005 , the third Vioxx case was ruled a mistrial when the jury could not come to a unanimous agreement on the verdict. Of the nine jurors, eight had voted that Merck was not guilty with one juror holding firm on Merck's guilt. The eight jurors who voted for Merck stated that they felt that Merck had made stronger scientific arguments supporting its case than the patient had; Irwin was already in a high-risk category for a heart attack. 34 The retrial for Irwin started on February 6, 2006, in New Orleans. After 2 weeks, the jury found Merck not responsible for the death of Irwin, giving the company its second legal win. By this time, more than 9,650 lawsuits had been filed against Merck. 35 Merck defended itself against another plaintiff in Rio Grande City, Texas, on January 25, 2006. The family of Leonel Garza Sr. sued Merck over his fatal heart attack in 2001. Garza had heart problems for more than 20 years and was a smoker. In addition, he also had a quadruple-bypass operation in the past. 6 On April 21,2006 , the Texas jury found Merck liable for the fatal heart attack of Garza. The jury awarded the Garza family $7 million in compensatory damages and $25 million in punitive damages. 37 On December 21 , 2006 , the $32 million award was reduced to $8.7 million by state law that caps punitive damages at $750,000.38 On March 6, 2006, the next Vioxx trial started in Atlantic City, with a patient who had a heart attack while using Vioxx but survived. The patient, Thomas Cona, had a heart attack in 2003 but was in a high-risk category to have a heart attack. Another patient, John McDarby, also had a heart attack and survived and was in a high-risk category for heart attacks. The lawyer for the plaintiff was Mark Lanier, who was the lawyer who won the first Vioxx case in Texas.. On April 5 , the jury in the MeDarby case found Merck guilty of not properly warming patients. The jury awarded McDarby $3 million in compensatory damages and his wife, Irma, $1.5 million. The jury also found Merck guilty of committing consumer fraud against both McDarby and Thomas. A decision on punitive damages was scheduled later by the court. 40 On April 10, 2006, the jury added $9 million in punitive damages to the decision pertaining to McDarby's case. 41 In April 2006, suing Merck became easier because of a "trial in a box" system that had been developed by lawyers. The system set up a step-by-step process in which smaller law firms could help their clients sue Merck. The package - which included select portions of video disposition, a trial theme grid that laid out the basic arguments in the case, and courtroom slides - was free to any law office, but lawyers who used the package had to pay a contingency fee between 3% and 6% of any rewards or settlements that were received from Merck. Shortly after it was introduced, almost 200 law firms had signed a contract to obtain the package. By using the package, the estimated cost of the trial for the law firm could be reduced to $50,000.42 In May 2006, Merck released data that showed that the risks of cardiovascular problems could occur starting at 4 months after first taking Vioxx instead of the 18 months, which was the cornerstone of its defense in its lawsuits. In the study called Approve, the data showed that patients taking Vioxx started to have more cardiovascular events than did those taking the placebo by the fourth month of the study. By this time, approximately 11,500 lawsuits had been filed against Merck..3 A study published by the Canadian Medical Association showed that senior patients who took Vioxx had the highest risk of a heart attack within the first 2 weeks of taking the drug, which countered Merck's defense that Vioxx had only long-term effects. 44 On May 11 , 2006, Merck released additional data that showed that patients who took Vioxx were at risk of having a heart attack or stroke for as long as 1 year after they stopped taking Vioxx. 45 The November 2000 New England Journal of Medicine article that highlighted the benefits and downplayed the risks of Vioxx might have had "lax" editing. The journal's executive editor, Gregory Curfman, admitted in a disposition that the authors might have been allowed to make misleading claims in the article because of a lax review of the article's results by the journal's editorial staff. Curfman also admitted that the Vioxx article generated between $697,000 and $836,000 in revenue from selling 929,400 reprints, which were mostly bought by Merck. 46 Merck received its fourth victory in seven verdicts when a New Jersey jury found that the company was not responsible for Elaine Doherty's heart attack. The jury stated that Merck had acted responsibly and the 68-year-old Doherty's doctor knew about the potential risk of taking Vioxx. . Merck was victorious in the next trial as well. A California jury also found that Merck was not responsible for the heart attack of a 71-year-old patient, Stewart Grossberg. The jury again cited that Merck had made the patient aware of the potential risks of taking Vioxx. 48 By August 2006, Merck was facing 14,000 lawsuits, but the recent victories in court led to the withdrawal of 300 federal cases in which the patients either had weak cases or could not prove that they had taken the drug. 49 In a double dose of reality, on August 17, 2006, Merck lost in two separate cases. A federal court ordered Merck to pay the plaintiff in one case, 62-year-old Gerald Barnett, \$51 million in compensatory and punitive damages. The jury found that Merck was negligent by failing to warn doctors about the risks linked to the drug. In addition, the jury stated that Merck had knowingly misrepresented or failed to properly disclose relevant information about Vioxx. 50 Furthermore, Merck's first Vioxx victory was thrown out because of the new questions pertaining to the data that was generated in previous Merck studies. 51 On August 30, 2006, a federal judge in New Orleans described the $51 million verdict for Barnett as "grossly excessive" and ordered a review of the assignment of damages in the case. The judge agreed with the jury that Merck should be liable for Barnett's heart attack but stated that issues such as lost wages should not be included in the damages because the plaintiff was able to go back to many of his regular daily activities. 52 One month later in September 2007, the Supreme Court of New Jersey rejected a class-action lawsuit against Merck for its Vioxx litigation. The court ruled that a nationwide class-action lawsuit was not appropriate. The ruling was a huge victory for Merck because the company was now allowed to continue to have each case tried separately instead of having the plaintiffs' resources be pooled in a class-action motion. The shares of Merck rose more than 2% to $50.47 after the ruling was announced. 64 In what could be considered one of the greatest legal bargains in corporate history, on November 8, 2007, Merck agreed to settle 27,000 Vioxx lawsuits covering approximately 47,000 plaintiffs for $4.85 billion or approximately $100,000 per lawsuit. Therefore, each plaintiff would receive just over $100,000 before legal fees and expenses, which can be equivalent to between 30% and 50% of the payment. As a result, the average plaintiff will receive between $50,000 and $70,000. 65 By March 2008 , 44,000 of the 47,000 plaintiffs had signed up to be part of the $4.85 billion settlement. 66 In February 2008, Merck agreed to pay $671 million to settle civil legal claims by the government that Merck had overcharged Medicaid health programs for four of its drugs. In addition, Merck was accused of offering doctors fees and gifts in exchange for the doctors prescribing Merck drugs. Merck's response was that there was just "a disagreement" over the rules of the Medicaid rebate program. Merck had drafted numerous research studies pertaining to Vioxx. In other words, Merck representatives had written the research papers and then gave credit to other authors. In one research paper for which Merck wanted to have a well-known researcher as the lead author, the lead author's name is not on the paper, which has the comment "External author?" instead. Merck acknowledged that it occasionally hired external medical writers to help draft research papers that would be given to doctors whose names would eventually appear on the article. Merck also stated that the authors of the paper were actively involved in the research or analysis of the data in the paper. The Journal of the American Medical Association (JAMA) examined published articles pertaining to Vioxx in which JAMA concluded, "It is clear that at least some of the authors played little direct roles in the study or review, yet still allowed themselves to be named as authors.. 68 In May 2008, Merck paid $58 million to settle civil claims that it down played the health risks of Vioxx in its marketing campaigns. In addition, Merck also agreed to submit all future television commercials to review by the FDA before they are aired for the next 7 years. 69 Also in May 2008, Carol Ernst's $26 million verdict from the first plaintiff Vioxx victory in 2005 was overturned b a state appeals court in Texas. The appeals court found that the plaintiffs had not proven that Vioxx caused Carol's husband's death. 70 In May 2009 , it was discovered that Merck subsidiary in Australia was paying the Australian officer of an academic publisher, Elsevier, from 2002 through 2005 to have eight positive compilations of scientific articles published about Vioxx. The article were published in the Australasian Journal of Bone and Joint Medicine. Elsevier did not disclose that Merck had paid to have the articles published, and readers perceived that the articles were based on peer-review evaluations, but they were not. 71 In April 2010, shareholders won a lawsuit against Merck that claimed that Merck executives and board members did not perform their fiduciary duties in marketing Vioxx. Merck agreed to pay as much as $12.15 million of the shareholders' legal fees to settle the case. In addition, Merck agreed to hire a chief medical officer to be responsible for monitoring product safety and marketing. 72 In November 2011, Merck agreed to pay $950 million in fines and civil settlements and to plead guilty to one criminal misdemeanor charge for its role in marketing and selling Vioxx. The criminal charge was based on the notion that Merck illegally promoted Vioxx to its patients. Merck had made inaccurate, unsupported, or misleading statements of patient safety when they used Vioxx. Merck pleaded guilty to introducing a "misbranded" product, Vioxx, into interstate commerce, which is a criminal violation of the Food, Drug, and Cosmetic Act. Merck agreed to pay a fine of \$321.6 million and \$628.4 million in civil lawsuit settlements. The civil settlement was allocated to pay \$426 million to the federal government and \$202 million to state Medicaid agencies that had sued Merck for civil claims. As a result, Merck has paid nearly $6 billion to settle lawsuits for a drug that generated total sales of $11 billion from 1999 to 2004.73 In 2012 , researchers at the University of Pennsylvania determined why using Vioxx can lead to heart problems. In order to relieve pain, Vioxx suppresses the production of a single enzyme, Cox-2, which is needed to help protect the heart. The blocking of the Cox-2 enzyme suppresses the production of molecules that are used to break up potential blood clots and increase the blood flow inside the body. 74 In August 2006 , Merck announced that it had developed a replacement for Vioxx called Arcoxia. Merck stated that it already had been sold in 62 other countries but had not received final approval from the FDA. The FDA had deferred a decision on the approval because it appeared that Arcoxia could have similar risks to the heart attacks that occurred with Vioxx. One of the side effects of Arcoxia was high blood pressure. On September 6,2006, an internal investigation found that Merck behaved in an appropriate manner as it related to Vioxx. At a cost of $21 million and having taken more than 20 months and 53,000 hours to complete, the report stated that Merck executives did not hide the potential risks to patients taking Vioxx. The report concluded that Merck officials took "reasonable steps" in researching the potential health risks of taking Vioxx. 53 With 14,200 lawsuits waiting to be tried, the report could have been a critical part of future litigation if it had found that Merck was guilty of negligence. Even with the clean bill of health from the report, Merck was facing an estimated legal liability of between $4 billion and $30 billion. 54 On September 12,2006, researchers from Harvard Medical School released the results of the summary of 114 clinical trials that examined the associated risks of using Vioxx. More than 116,000 people were part of the trials, and the results showed that Vioxx increased both kidney disease and arrhythmia risks. Furthermore, the arrhythmia risks were shown to have started soon after the treatment had started. 55 On September 26, 2006, a federal jury found Merck not responsible for the heart attack of a Kentucky plaintiff. After 3 hours of deliberation, the jury found the plaintiff had not proved that Vioxx was the cause of his heart attack 31/2 years earlier. 56 By the end of September 2006, Merck had 22,000 Vioxx-related lawsuits filed against it. Part of the surge in the number of lawsuits was because a number of states have a 2-year statute of limitations for plaintiffs to file lawsuits, which expired at the end of September, corresponding with the 2-year anniversary of Vioxx's withdrawal from the marketplace. 57 By September 30, 2006, Vioxx had 23,800 lawsuits that involved 41,750 plaintiffs. The company also faced 275 class-action lawsuits and stated that it would increase its reserves for Vioxx-related legal costs from $685 million to $958 million. 58 In the 11th trial related to Vioxx, Merck recorded another victory when a jury spent just 90 minutes in teliberation to decide that Merck was not responsible for Charles Mason's heart attack. Merck successfully argued that Mason's clogged arteries, rather than the use of Vioxx for 10 months, caused the heart attack. That made the tally seven victories and four losses for Merck; the 11 th case was an initial victory for Merck, but the decision was overturned. 59 In another victory for Merck, a federal judge ruled that the thousands of federal lawsuits against Merck could not be grouped into one national class-action lawsuit. Merck wanted to have each of the cases tried independently to highlight the unique characteristics of each plaintiff's complaint. 60 In an apparent increased level of momentum, on December 13,2006 , Merck also won its 12 th case in New Orleans when a jury decided in less than 2 hours that Merck had given adequate warning of the heart risks associated with the drug. By December 2006 , the numbers of outstanding lawsuits against Merck had climbed to 27,000 . Two days later, Merck won another victory when a jury in Alabama deliberated for less than 2 hours to decide that Merck was not accountable for Gary Albright's heart attack. 62 By August 2007, Merck had spent more than $1 billion in legal fees to defend itself in Vioxx cases. As part of its legal strategy, Merck automatically appealed any judgment against it, which gave Merck a potential additional opportunity to present its case while not being required to make any payments in judgments against the company until after the appeal. Thus, 2 years after Carol Ernst won the first Vioxx case against Merck for the death of her husband Robert, neither she nor any of the 45,000 other plaintiffs had received any money from Merck. In the 2 years after the initial legal judgment against Merck, its stock price increased by 80%, and the estimated legal liability for Merck had decreased from $25 billion to $5 billion. The legal counsel responsible for Merck strategy pertaining to the lawsuits, Kenneth Frazier, was promoted to president of global health division, which is in charge of Merck's marketing and sales forces and includes approximately 30,000 employees. 63