Question: really need help with part e) Question 2 (20pts): Use the standard approach to calculate the equilibrium concentrations of the all ions that that result
 really need help with part e)
really need help with part e)
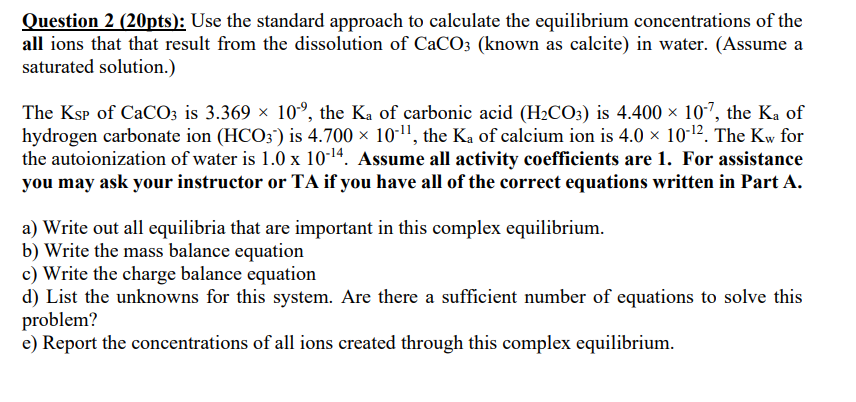
Question 2 (20pts): Use the standard approach to calculate the equilibrium concentrations of the all ions that that result from the dissolution of CaCO3 (known as calcite) in water. (Assume a saturated solution.) The Ksp of CaCO3 is 3.369 10-9, the Ka of carbonic acid (H2CO3) is 4.400 x 10-7, the Ka of hydrogen carbonate ion (HCO3') is 4.700 10-11, the K, of calcium ion is 4.0 10-12. The Kw for the autoionization of water is 1.0 x 10-14. Assume all activity coefficients are 1. For assistance you may ask your instructor or TA if you have all of the correct equations written in Part A. a) Write out all equilibria that are important in this complex equilibrium. b) Write the mass balance equation c) Write the charge balance equation d) List the unknowns for this system. Are there a sufficient number of equations to solve this problem? e) Report the concentrations of all ions created through this complex equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


