Question: Really need some help please! Pure Solvent 80513 Enter the mass of lauric acid (9) Benzoic Acid Solution 1.0566 Enter the mass of benzoic acid
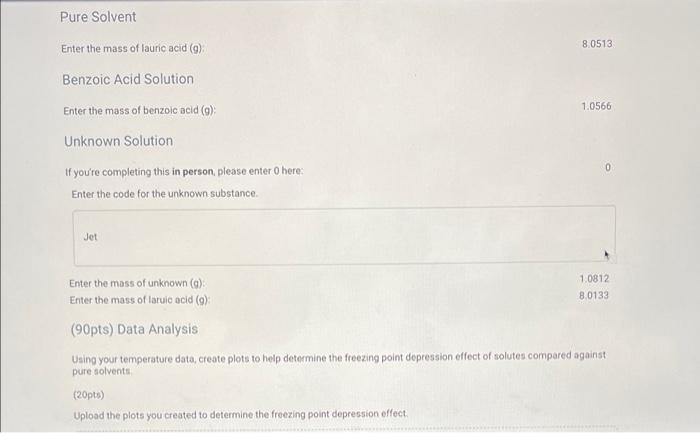
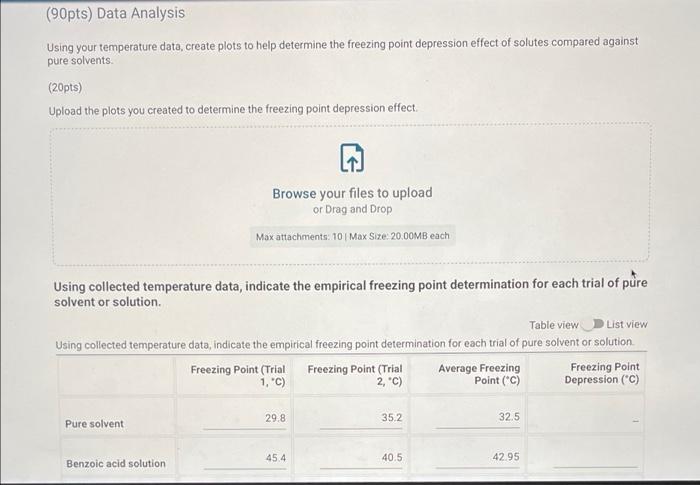
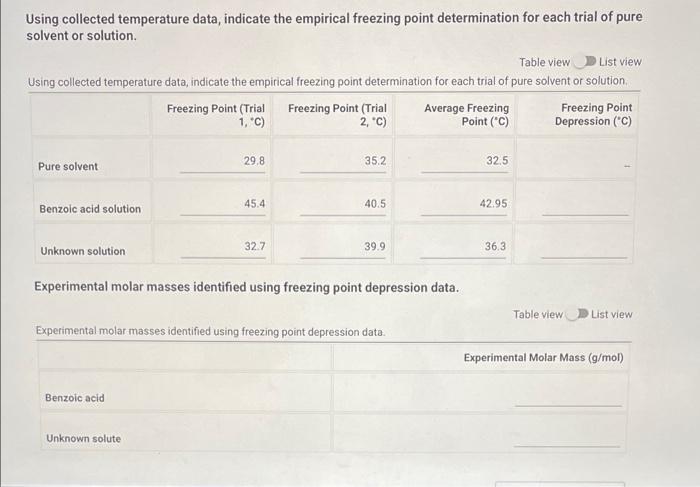
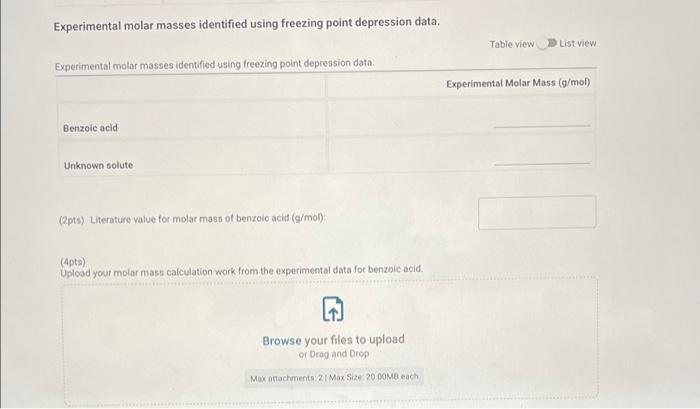
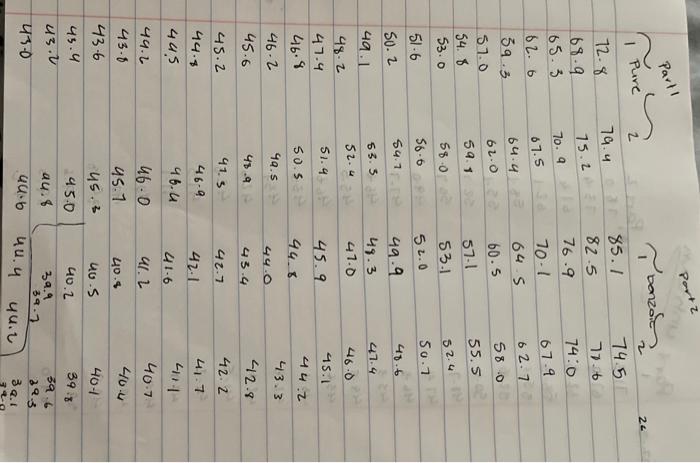
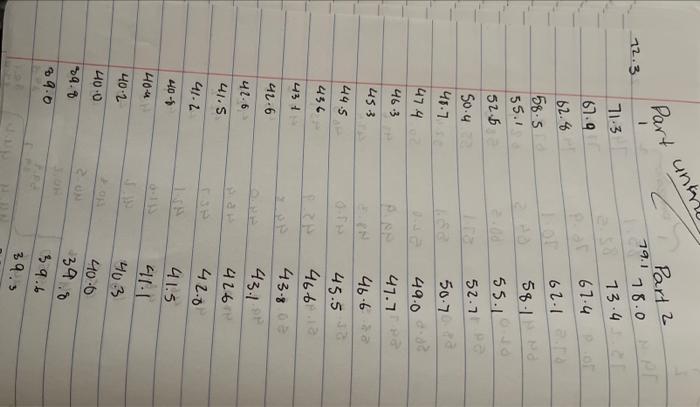
Pure Solvent 80513 Enter the mass of lauric acid (9) Benzoic Acid Solution 1.0566 Enter the mass of benzoic acid (9) Unknown Solution 0 If you're completing this in person, please enter O here: Enter the code for the unknown substance Jet Enter the mass of unknown (0) 1.0812 Enter the mass of laruic acid (9) 8.0133 (90pts) Data Analysis Using your temperature data, create plots to help determine the freezing point depression effect of solutes compared against pure solvents (20pts) Upload the plots you created to determine the freezing point depression effect (90pts) Data Analysis Using your temperature data, create plots to help determine the freezing point depression effect of solutes compared against pure solvents (20pts) Upload the plots you created to determine the freezing point depression effect. Browse your files to upload or Drag and drop Max attachments: 10 Max Size 20.00MB each Using collected temperature data, indicate the empirical freezing point determination for each trial of pure solvent or solution. Table view List view Using collected temperature data, indicate the empirical freezing point determination for each trial of pure solvent or solution Freezing Point (Trial Freezing Point (Trial Average Freezing Freezing Point 1, "C) 2.c) Point (C) Depression (C) 29.8 35.2 32.5 Pure solvent 45.4 40.5 42.95 Benzoic acid solution Using collected temperature data, indicate the empirical freezing point determination for each trial of pure solvent or solution Table view List view Using collected temperature data, indicate the empirical freezing point determination for each trial of pure solvent or solution Freezing Point (Trial Freezing Point (Trial Average Freezing Freezing Point 1, "C) 2,"C) Point (C) Depression (C) 29.8 Pure solvent 352 32.5 45.4 40.5 Benzoic acid solution 42.95 32.7 Unknown solution 39.9 36.3 Experimental molar masses identified using freezing point depression data. Table view List view Experimental molar masses identified using freezing point depression data. Experimental Molar Mass (g/mol) Benzoic acid Unknown solute Experimental molar masses identified using freezing point depression data. Table view List view Experimental molar masses identified using freezing point depression data, Experimental Molar Mass (g/mol) Benzoic acid Unknown solute (2pts) Literature value for molar mass of benzoic acid (g/mol); (apta) Upload your molar mass calculation work from the experimental data for benzoic acid Browse your files to upload or Drag and Drop Max attachments: 2 Max Size 20.00MB each (2pts) Percent error for molar mass of benzoic acid (%) (4pts) Upload your work for calculating the percent error of molar mass of benzoic acid. Browse your files to upload or Drag and Drop Max attachments: 2 Max Size: 20.00MB each (Apts) Upload your work for calculating the molar mass of the unknown solute Browse your files to upload or Drag and Drop Max attachments: 21 Max Size: 20.00MB each (ots) Orgonorop Max attachments: 2 Max Size: 20.00MB each (4pts) Upload your work for calculating the molar mass of the unknown solute Browse your files to upload or Drag and drop Max attachments: 2 Max Size: 20.00MB each (4pts) In complete sentences, discuss the percent error in the molar mass determination for benzoic acid. In the experiment what do you observe that might lead to error in the results? Be thorough Normal BIU X1 X1 EEE lli 7 Parta Parti Pure 24 72.8 68.9 165.3 67.9 62.b 59.3 57.0 |54.8 53.0 51-6 T senza 2 74.485./ 74.5 75.2 E 82.5 77.6 70. 76.9 74:0 67.5 70.1 64.422 645 62.7 60.5 580 57-1 55.5 53.1 52.0 50.1 49. 48.6 63.5 48.3 47.4 52. 41.0 61.0.22 59.13 58.0 56.6 54.1:. 52.4 30. 2 51.4 4.1 48.2 47.4 46.8 46.2 45.6 60.5 40.5 4.4 45.2 44.4 91.3 46.9 46.4 46.0 45.1 45.9 44. 44. 43.3 44. 42.8 45.4 42.2 41.7 41.1 42.1 411 41-6 40.7 4. 40.4 40.4 40.5 40-1 40.2 39. 34.1 19.3 .4 4u.t 3. 445 44. 43.8 43.6 460 45.1 45. 45.0 44. 40.6 45.4 3.0 39. . untral Part 19.1 72.3 Part 2 78.0 NPR 73.4 71-3 67.4 62.6 158.5 55.1 67.4 62-1 58-1 55.1 52.5 50.4 52.7.pe 50-7 48.7 474 463 DJa d. 49.0 47.7 Pa 453 46.62 44.5 45.51a 456 4661 431 43.80 42.6 43.18 41.6 41.5 416 42.0 41.2 40+ 40-4 40-2 40-0 Jo 41.5 411 103 40-6 346 9.. 39.5 64.6 29.0 2- 3.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


