Question: Rederive the simple Stefan problem result (Eqn. 3.40) using the molar flux form of Fick's law (Egn. 3.6) with boundary conditions expressed in mole fractions.

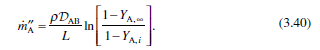
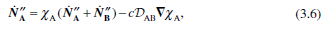
Rederive the simple Stefan problem result (Eqn. 3.40) using the molar flux form of Fick's law (Egn. 3.6) with boundary conditions expressed in mole fractions. Note that the molar concentration c(= PIRT) is constant for an ideal gas, unlike the density p, which depends on the mixture mo- lecular weight. Leave the final result in terms of the mole fractions. 1-YA. . PD AB ma -In (3.40) L 1-Yri NK = XAN+ND)-CDAB TXA (3.6)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


