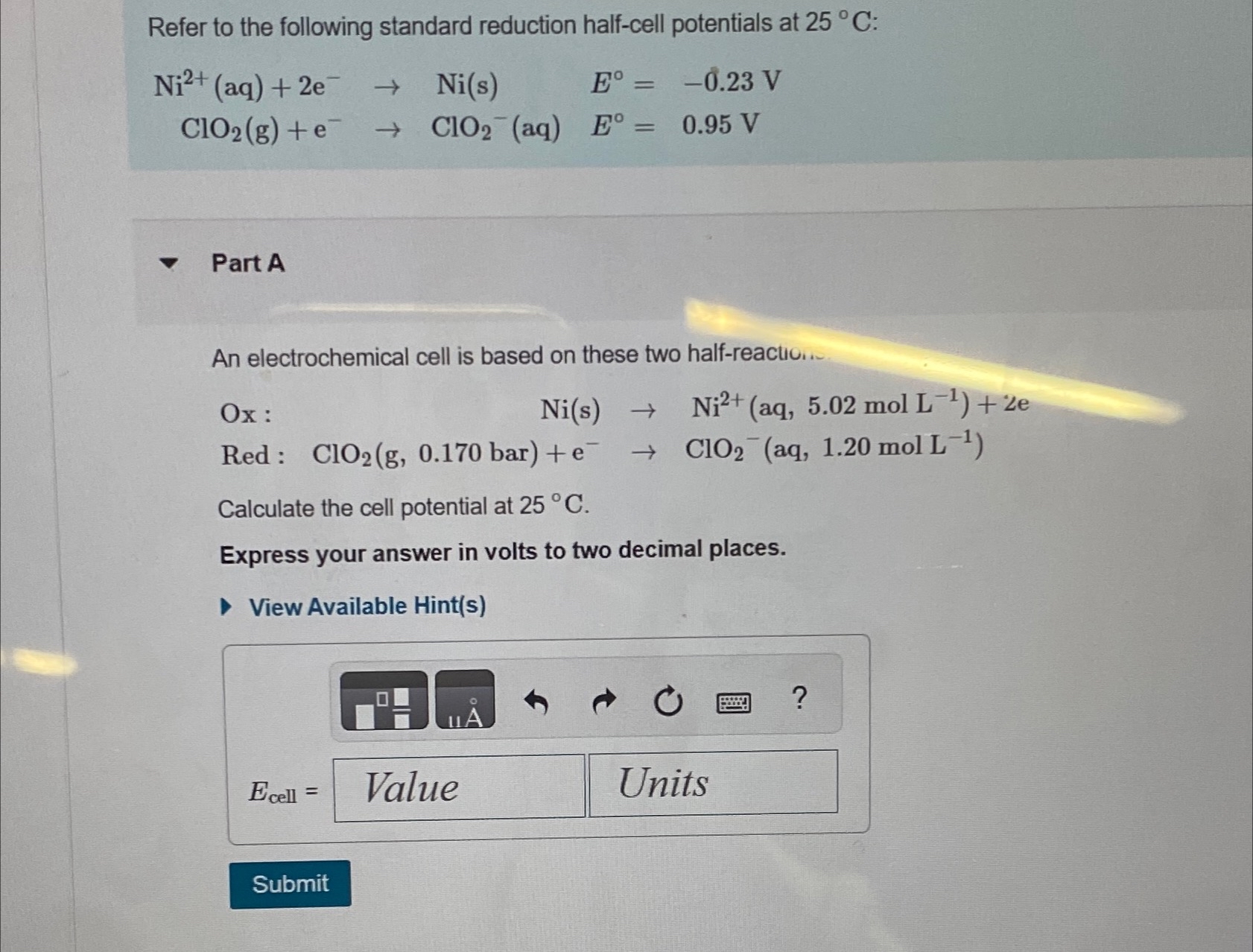
Question: Refer to the following standard reduction half - cell potentials at 2 5 C : N i 2 + ( a q ) + 2
Refer to the following standard reduction halfcell potentials at :
Part A
An electrochemical cell is based on these two halfreactio...
:
Red:
Calculate the cell potential at
Express your answer in volts to two decimal places.
View Available Hints
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


