Question: Rel. Intensity (+) A B C E On the above graphic, which structure best represents the fragment at m/z 41. C, Because it has the
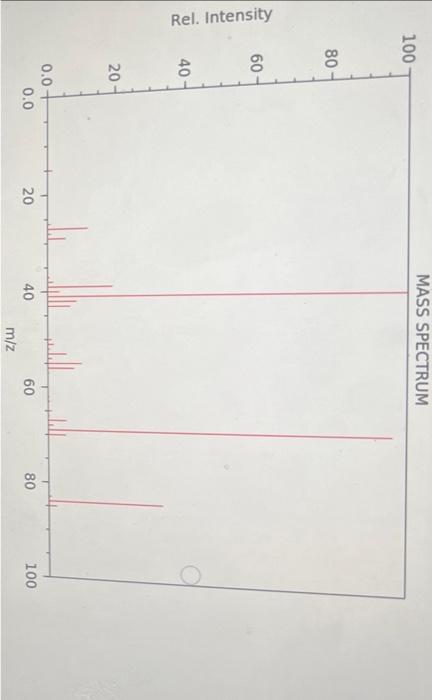
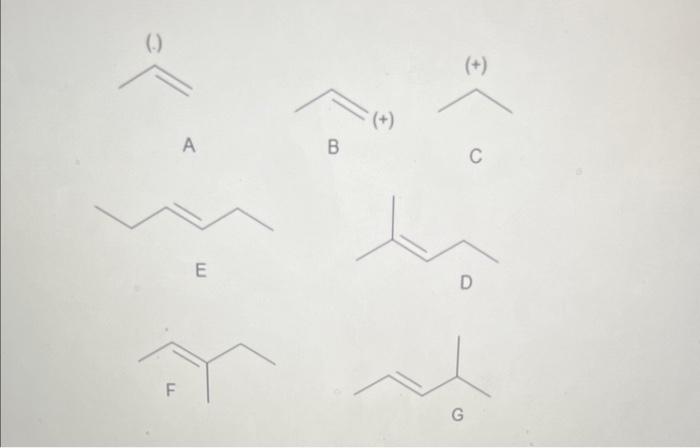

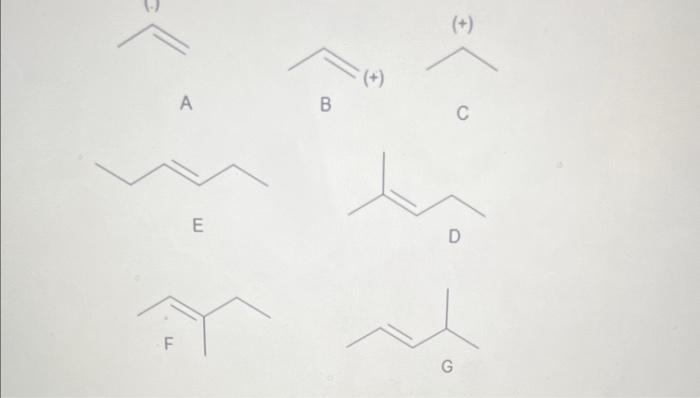

Rel. Intensity (+) A B C E On the above graphic, which structure best represents the fragment at m/z 41. C, Because it has the correct mass and carries a positive charge. B, Because it has the correct mass and carries a positive charge. A, Because it has the correct mass and carries a negative charge. E Taking into account the correct answers to the previous relevant questions, on the above graphic, which structure best represents the structure of the unknown molecule? Justify your answer. F, because it accounts for the fragments at m/z69 and 41 without losing a neutral ethyl group G, because it accounts for the fragments at m/z69 and 41 without losing a neutral ethyl group D, because it accounts for the fragments at m/z69 and 41 without losing a neutral ethyl group Rel. Intensity (+) A B C E On the above graphic, which structure best represents the fragment at m/z 41. C, Because it has the correct mass and carries a positive charge. B, Because it has the correct mass and carries a positive charge. A, Because it has the correct mass and carries a negative charge. E Taking into account the correct answers to the previous relevant questions, on the above graphic, which structure best represents the structure of the unknown molecule? Justify your answer. F, because it accounts for the fragments at m/z69 and 41 without losing a neutral ethyl group G, because it accounts for the fragments at m/z69 and 41 without losing a neutral ethyl group D, because it accounts for the fragments at m/z69 and 41 without losing a neutral ethyl group
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


