Question: Reminder: the relation between theoretical yiled (T.Y), actual yield (AY) and percent yield (%Y) is as follows: TY=100(AY/%Y) What is the percent yield of 1,4-di-t-butyl-2,5-dimethoxybenzene
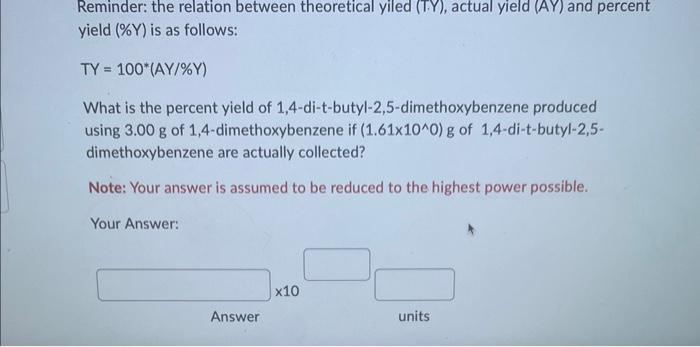
Reminder: the relation between theoretical yiled (T.Y), actual yield (AY) and percent yield (%Y) is as follows: TY=100(AY/%Y) What is the percent yield of 1,4-di-t-butyl-2,5-dimethoxybenzene produced using 3.00g of 1,4 -dimethoxybenzene if (1.61100)g of 1,4 -di-t-butyl-2,5dimethoxybenzene are actually collected? Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: Answer units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


