Question: Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the average atomic mass
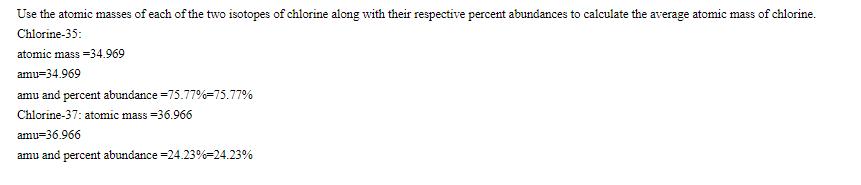
Use the atomic masses of each of the two isotopes of chlorine along with their respective percent abundances to calculate the average atomic mass of chlorine. Chlorine-35: atomic mass -34.969 amu=34.969 amu and percent abundance -75.77%-75.77% Chlorine-37: atomic mass =36.966 amu=36.966 amu and percent abundance -24.23%-24.23%
Step by Step Solution
★★★★★
3.52 Rating (145 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

To calculate the average atomic mass of chlorine we can use ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


