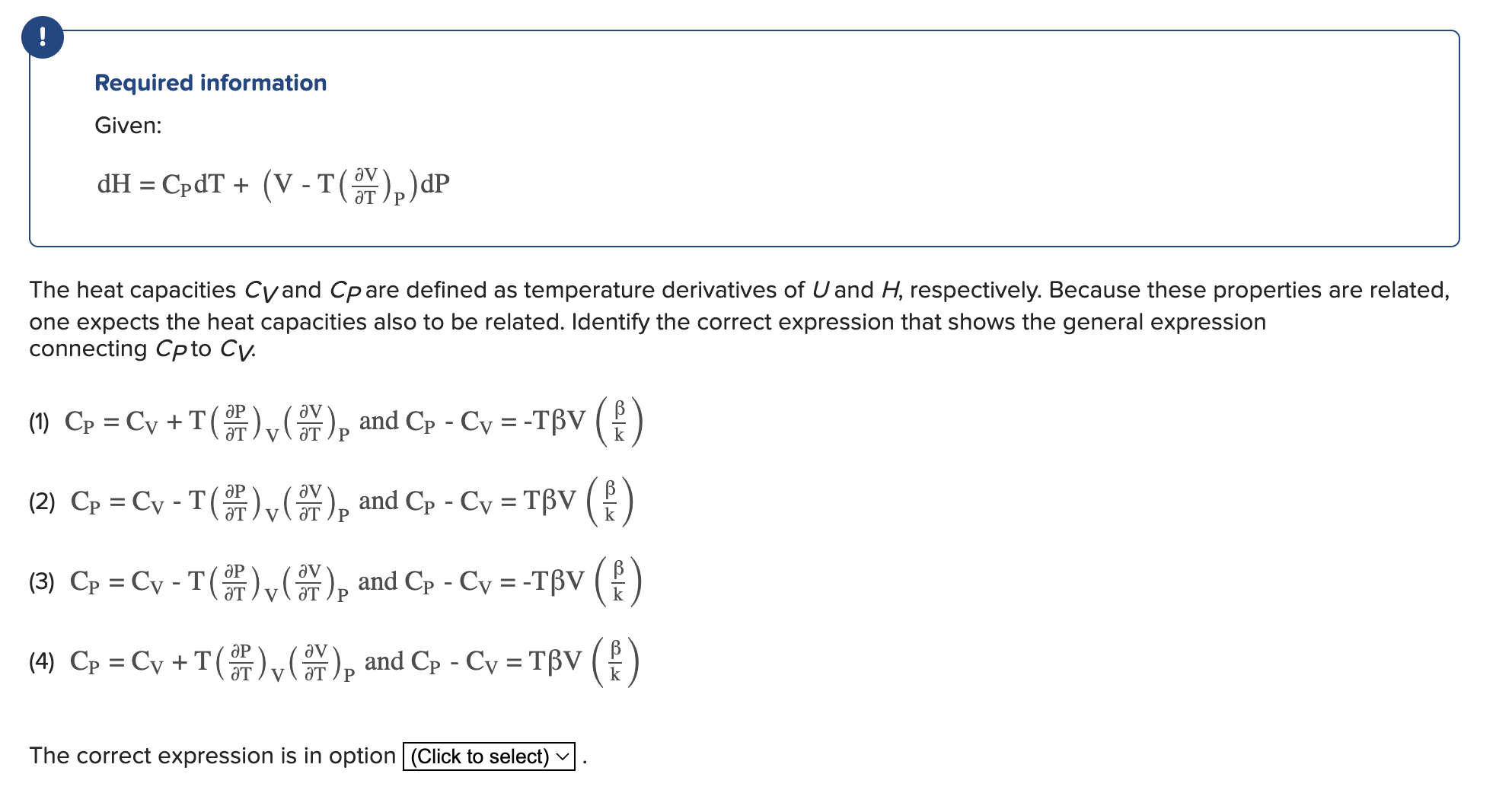
Question: Required information Given: d H = C P d T + ( V - T ( d e l V d e l T )
Required information
Given:
The heat capacities and are defined as temperature derivatives of and respectively. Because these properties are related,
one expects the heat capacities also to be related. Identify the correct expression that shows the general expression
connecting to
and
and
and
and
The correct expression is in option
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


