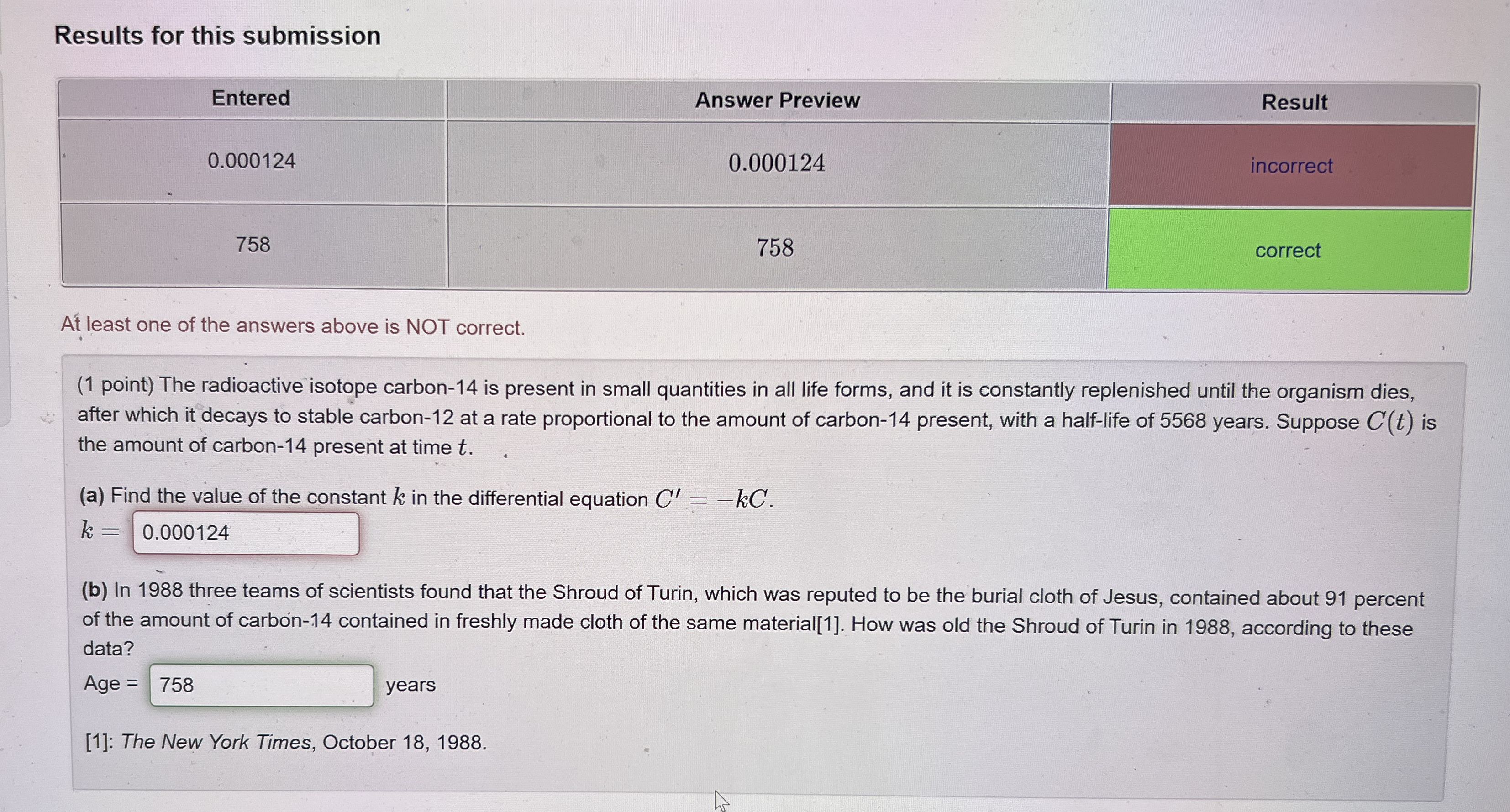
Question: Results for this submission table [ [ Entered , Answer Preview,Result ] , [ 0 . 0 0 0 1 2 4 , 0
Results for this submission
tableEnteredAnswer Preview,Resultincorrectcorrect
At least one of the answers above is NOT correct.
point The radioactive isotope carbon is present in small quantities in all life forms, and it is constantly replenished until the organism dies, after which it decays to stable carbon at a rate proportional to the amount of carbon present, with a halflife of years. Suppose is the amount of carbon present at time
a Find the value of the constant in the differential equation
b In three teams of scientists found that the Shroud of Turin, which was reputed to be the burial cloth of Jesus, contained about percent of the amount of carbon contained in freshly made cloth of the same material How was old the Shroud of Turin in according to these data?
Age years
: The New York Times, October
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


