Question: Reversible equilibria Reversible equilibria are very important for understanding biochemical systems. The equation for reversible oxygen binding by myoglobin provides an example. See fig. (a)
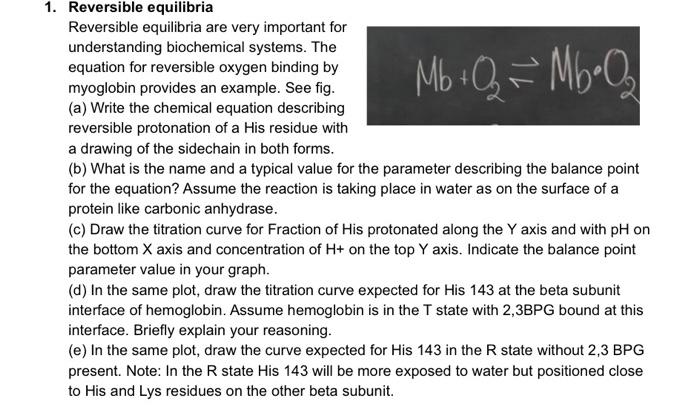
Reversible equilibria Reversible equilibria are very important for understanding biochemical systems. The equation for reversible oxygen binding by myoglobin provides an example. See fig. (a) Write the chemical equation describing reversible protonation of a His residue with a drawing of the sidechain in both forms. (b) What is the name and a typical value for the parameter describing the balance point for the equation? Assume the reaction is taking place in water as on the surface of a protein like carbonic anhydrase. (c) Draw the titration curve for Fraction of His protonated along the Y axis and with pH or the bottom X axis and concentration of H+ on the top Y axis. Indicate the balance point parameter value in your graph. (d) In the same plot, draw the titration curve expected for His 143 at the beta subunit interface of hemoglobin. Assume hemoglobin is in the T state with 2,3BPG bound at this interface. Briefly explain your reasoning. (e) In the same plot, draw the curve expected for His 143 in the R state without 2,3 BPG present. Note: In the R state His 143 will be more exposed to water but positioned close to His and Lys residues on the other beta subunit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


