Question: Review Classify each solid as a covalent, ionic, metallic, or molecular solid. Drag the appropriate items to their respective bine. View Available Hint(a) Chemists use
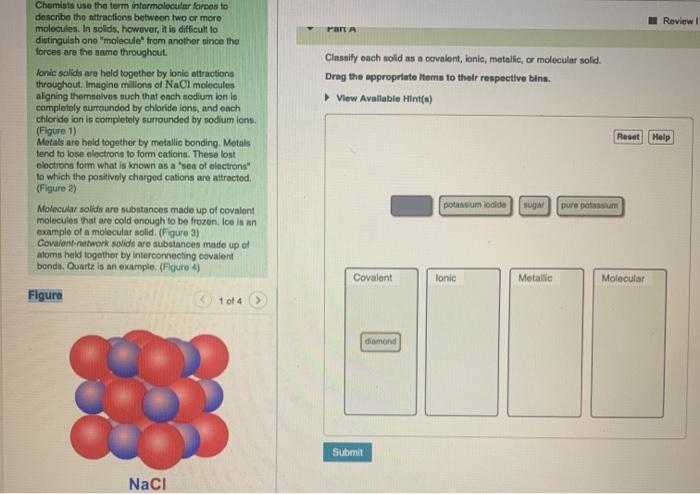
Review Classify each solid as a covalent, ionic, metallic, or molecular solid. Drag the appropriate items to their respective bine. View Available Hint(a) Chemists use the term intermolecular forces to describe the attractions between two or more molecules. In solids, however, it is difficult to distinguish ono "molecule from another since the forces are the same throughout Tonic solids are held together by lonic attractions throughout. Imagine millions of NaCl molecules aligning themselves such that onch sodium lon lo completely surrounded by chloride ions, and onch chloride ion is completely surrounded by nodium ions. (Figure 1) Metals are held together by metallic bonding Metals tend to lose electrons to form cations. These lost electrons form what is known as a 'sea of electrons" to which the positively charged cations are attracted. (Figure 2) Molecular solids are substances made up of covalent molecules that are cold enough to be frozen Ice is an example of a molecular solid. (Figure 3) Covalent network solids are substances made up of atoms held together by interconnecting covalent bondo. Quartz is an example: (Figure 1) Reset Help potassium odide SUGA pure potassium Covalent Ionic Metallic Molecular Figure 10t4 diamond Submit NaCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


