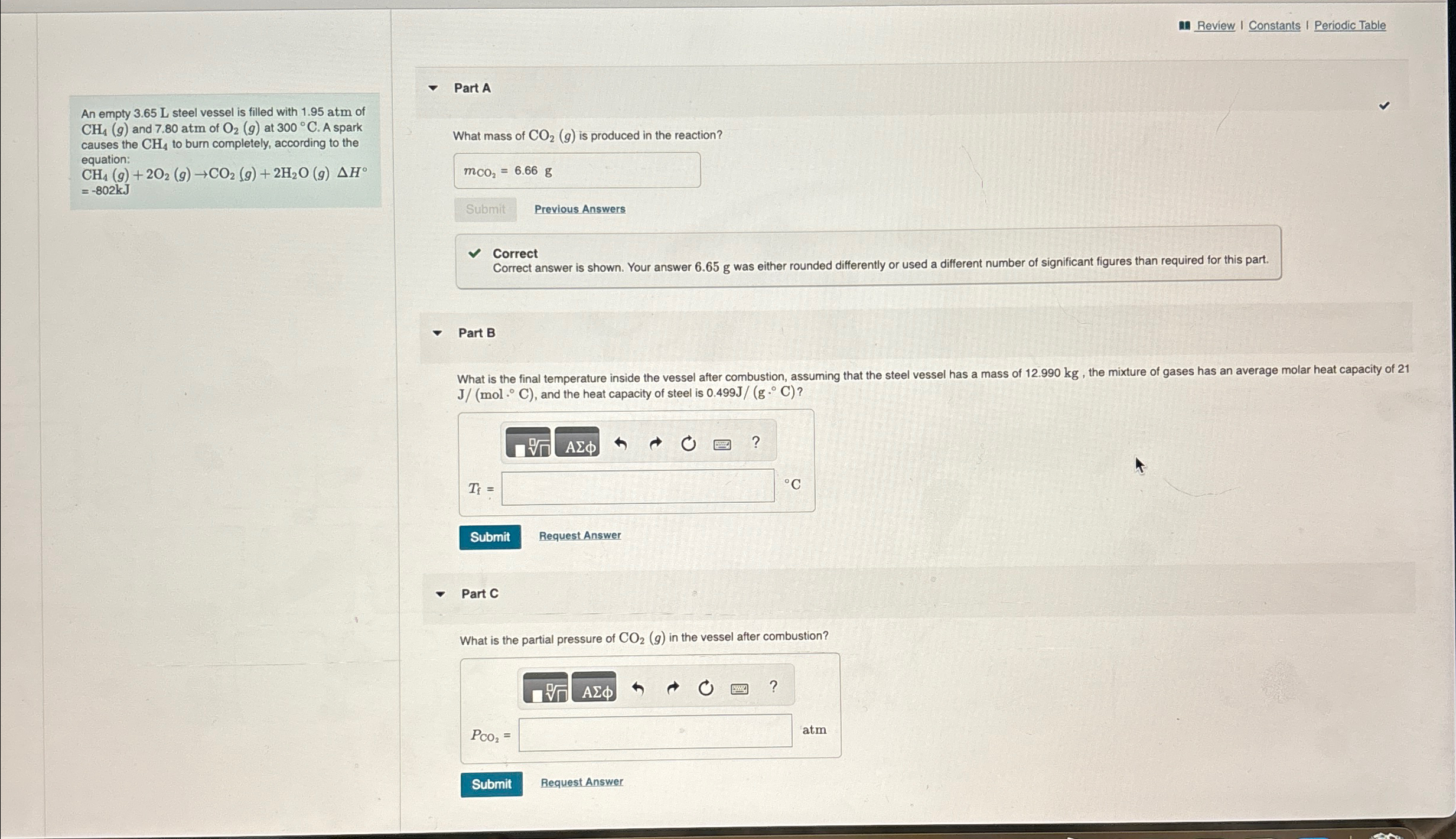
Question: Review I Constants I Periodic Table An empty 3 . 6 5 L steel vessel is filled with 1 . 9 5 atm of C
Review I Constants I Periodic Table
An empty steel vessel is filled with atm of and atm of at A spark causes the to burn completely, according to the equation:
Part A
What mass of is produced in the reaction?
Previous Answers
Correct
Correct answer is shown. Your answer was either rounded differently or used a different number of significant figures than required for this part.
Part B
What is the final temperature inside the vessel after combustion, assuming that the steel vessel has a mass of the mixture of gases has an average molar heat capacity of and the heat capacity of steel is
Request Answer
Request Answer
Part C
What is the partial pressure of in the vessel after combustion?
Request Answer
please answer both, thank you so much!!
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


