Question: [Review Topical (Reference Pentane CsH2) and hexane CH..) form an ideal solution. At 25C the vapor pressures of pentane and hexane are 511 and 150
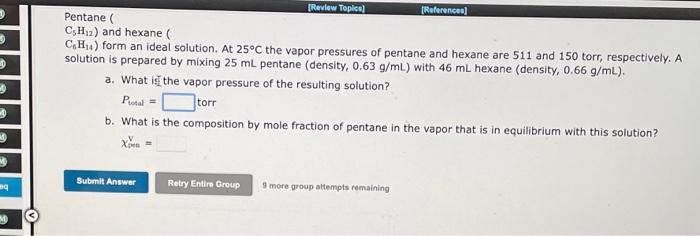
[Review Topical (Reference Pentane CsH2) and hexane CH..) form an ideal solution. At 25C the vapor pressures of pentane and hexane are 511 and 150 torr, respectively. A solution is prepared by mixing 25 mL pentane (density, 0.63 g/mL) with 46 mL hexane (density, 0.66 g/mL). a. What is the vapor pressure of the resulting solution? Potal b. What is the composition by mole fraction of pentane in the vapor that is in equilibrium with this solution? torr Submit Answer Retry Entire Group 9 more group attempts remaining M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


