Question: [Review Topics) RE Use the References to access important values if need Given the standard enthalpy changes for the following two reactions: (1) N2(g) +
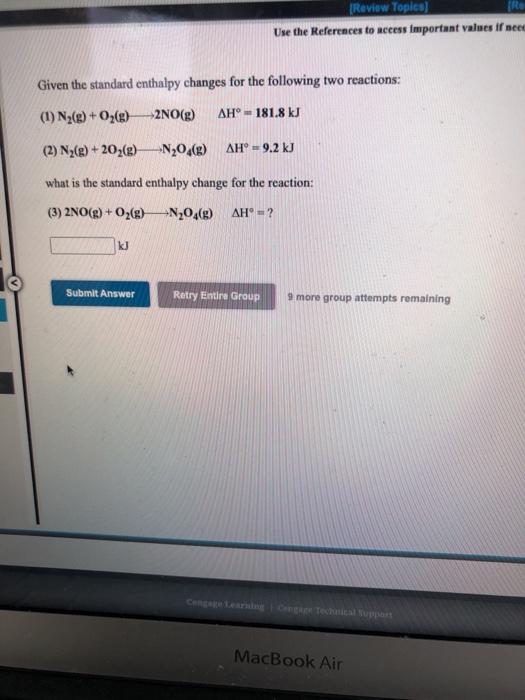
[Review Topics) RE Use the References to access important values if need Given the standard enthalpy changes for the following two reactions: (1) N2(g) + O2(8) -2NO(g) AH 181.8 kJ (2) N2(e) +202(g) -N204(8) AH = 9.2 kJ what is the standard enthalpy change for the reaction: (3) 2NO(g) + O2(8) N204(g) AH = ? kJ Submit Answer Retry Entire Group 9 more group attempts remaining MacBook Air
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


