Question: Roi Constanta Ponodic Table Part A Identify the expression for the rate of the reaction with respect to each of the reactants and products MISSED
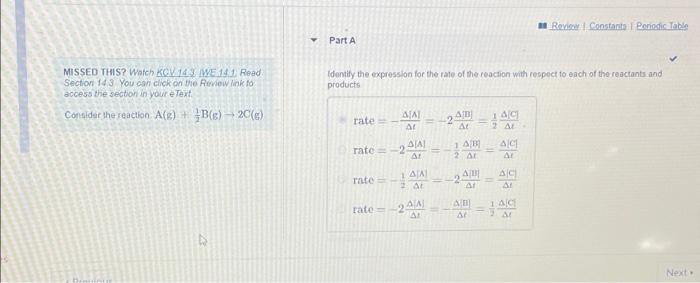
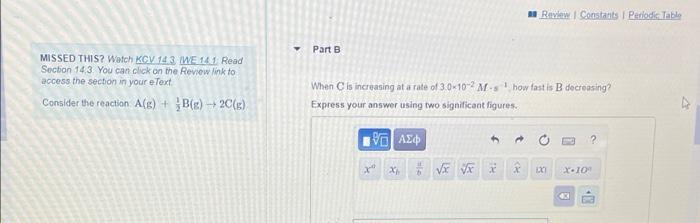
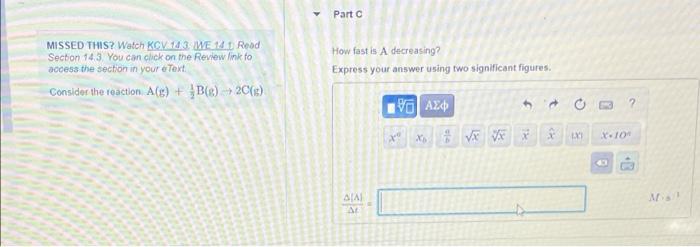
Roi Constanta Ponodic Table Part A Identify the expression for the rate of the reaction with respect to each of the reactants and products MISSED THIS? Watch KOV 14.3. WE 14.1. Read Section 143 You can click on the Review link to access the section in your e Text Condidat the reaction ACE) + BCE), 20(8) AC rate - SIA AL 2.40 1 rate = -20 AICI AI 2 AT rate = AL 2 AI AC A 1AC rate 2.000 Next - Review Constants Periodic Table Part B MISSED THIS? Watch KCV143 WE 141 Read Section 14.3 You can click on the Review link to access the section in your e Text Consider the reaction. A(E) + BC) - 20(6) When is increasing at a rate of 30-10-2M show fast is B decreasing Express your answer using two significant figures. ? XV VE * X-10 Part MISSED THIS? Watch KGV3 WE 1 Read Section 143. You can click on the Review link to access the section in your e Text Consider the reaction (s) + B8) 20() How fast is A decreasing? Express your answer using two significant figures FAXD ho XV X. 104 SIA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


