Question: Rotational Motion and the Hydrogenic Atom (40 points): a) What are the 3 quantum numbers for atomic orbitals and what do they represent? b) Where
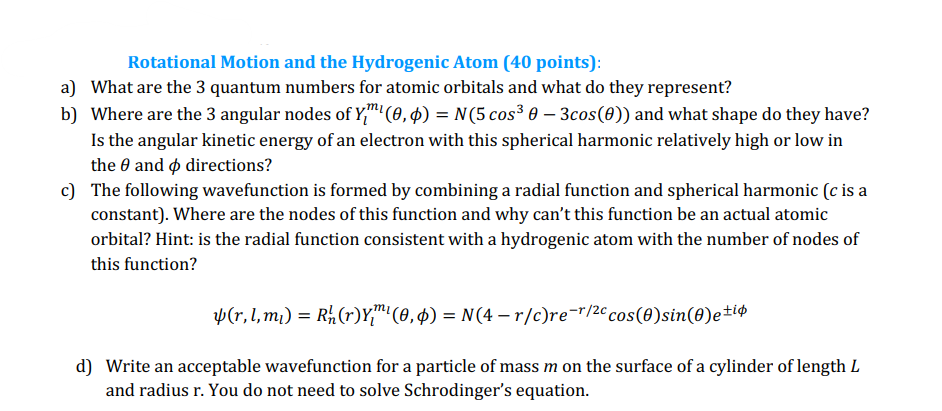
Rotational Motion and the Hydrogenic Atom (40 points): a) What are the 3 quantum numbers for atomic orbitals and what do they represent? b) Where are the 3 angular nodes of Y" (0,0) = N(5 cos3 0 - 3cos(O)) and what shape do they have? Is the angular kinetic energy of an electron with this spherical harmonic relatively high or low in the 6 and 0 directions? c) The following wavefunction is formed by combining a radial function and spherical harmonic (c is a constant). Where are the nodes of this function and why can't this function be an actual atomic orbital? Hint: is the radial function consistent with a hydrogenic atom with the number of nodes of this function? (r, l, mi) = Rh (r)YM! (0,0) = N(4 r/c)re=r/2c cos(Osin()eti d) Write an acceptable wavefunction for a particle of mass m on the surface of a cylinder of length L and radius r. You do not need to solve Schrodinger's equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


