Question: RUSH! Will upvote! A 75.0mL sample containing Fe3+ and Cu2+ required 28.45mL of 0.04186 M EDTA for complete titration. A 150.0mL sample of the unknown
RUSH! Will upvote!
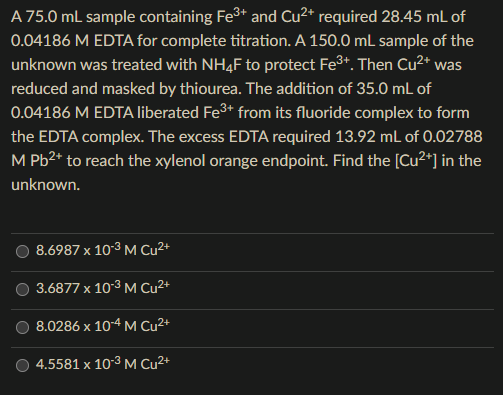
A 75.0mL sample containing Fe3+ and Cu2+ required 28.45mL of 0.04186 M EDTA for complete titration. A 150.0mL sample of the unknown was treated with NH4F to protect Fe3+. Then Cu2+ was reduced and masked by thiourea. The addition of 35.0mL of 0.04186 M EDTA liberated Fe3+ from its fluoride complex to form the EDTA complex. The excess EDTA required 13.92mL of 0.02788 MPb2+ to reach the xylenol orange endpoint. Find the [Cu2+] in the unknown. 8.6987103MCu2+3.6877103MCu2+8.0286104MCu2+4.5581103MCu2+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


