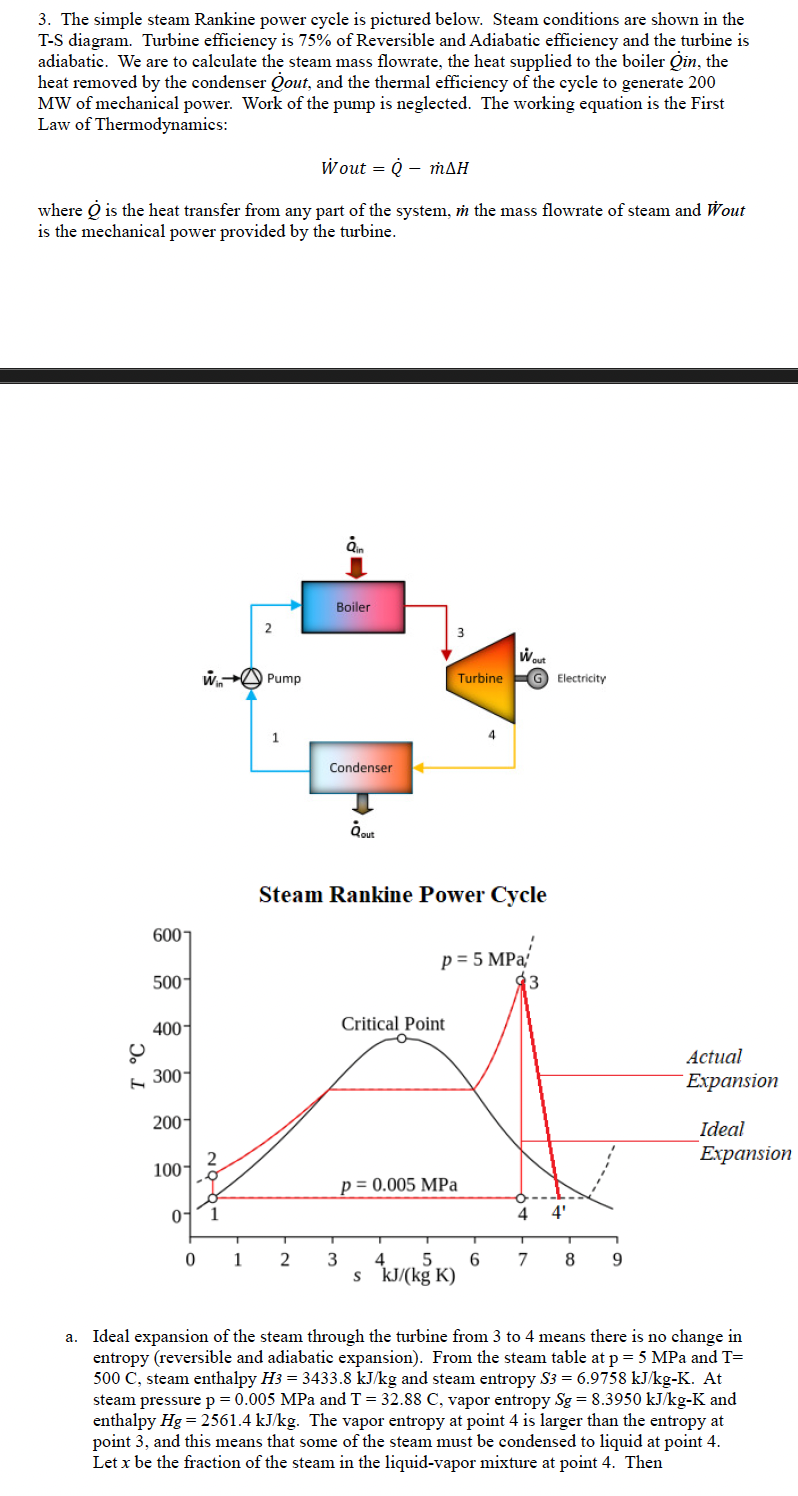
Question: S 4 = S 3 = ( 1 - x ) S f + x S g where S f = 0 . 5 7
where and are the entropy values of the
saturated liquid and vapor steam, respectively at MPa. The equation for can
be solved for steam fraction At MPa enthalpy of the liquid
and vapor and knowing we can calculate
Then is the enthalpy change at constant entropy. The
actual change in entropy and is the given
turbine efficiency.
Use the above information and the given out for the mechanical power of turbine
calculate the mass flowrate of the steam from the First Law of Thermodynamics.
Answer:
b Heat is added from point to point in the diagram. If the pump is adiabatic and
reversible the entropy at point is the same as the entropy at point At point the
entropy of the saturated liquid and enthalpy At
point pressure MPa and at the same entropy as point the enthalpy
Then from the mass flowrate calculate the heat added in the boiler:
Answer: Qin
c Heat is removed from the condenser from points to point There is an entropy
increase from point to point because the turbine expansion is less than isentropic
The enthalpy can be calculated from in part a Then calculate
Qout
Answer: Qout
d Calculate the cycle efficiency power outheat inAnswer: adiabatic We are to calculate the steam mass flowrate, the heat supplied to the boiler the
heat removed by the condenser out, and the thermal efficiency of the cycle to generate
MW of mechanical power. Work of the pump is neglected. The working equation is the First
Law of Thermodynamics:
out
where is the heat transfer from any part of the system, the mass flowrate of steam and out
is the mechanical power provided by the turbine.
Steam Rankine Power Cycle
a Ideal expansion of the steam through the turbine from to means there is no change in
entropy reversible and adiabatic expansion From the steam table at MPa and
C steam enthalpy and steam entropy At
steam pressure MPa and vapor entropy and
enthalpy The vapor entropy at point is larger than the entropy at
point and this means that some of the steam must be condensed to liquid at point
Let be the fraction of the steam in the liquidvapor mixture at point Then
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


