Question: s Le Chapter 14 homework.pdf - Adobe Acrobat Header DC File Edit View Sign Window Help Home Tools Chapter 14 Powere... Chapter 14 homew... *
s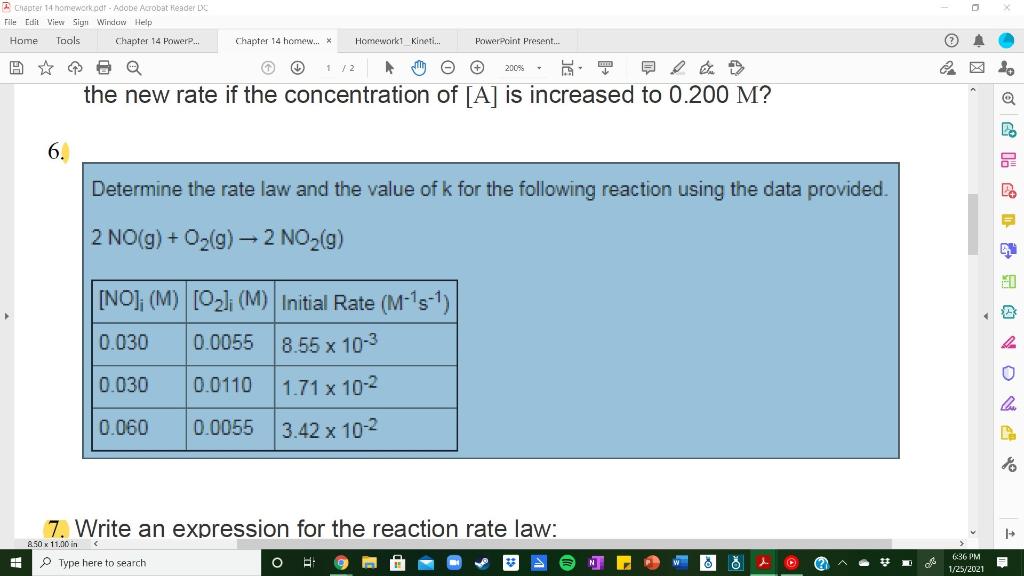
Le Chapter 14 homework.pdf - Adobe Acrobat Header DC File Edit View Sign Window Help Home Tools Chapter 14 Powere... Chapter 14 homew... * Homeworkt Kineti... PowerPoint Present.. 200% 6 the new rate if the concentration of [A] is increased to 0.200 M? 6. Determine the rate law and the value of k for the following reaction using the data provided. 2 NO(g) + O2(g) 2 NO2(9) HD [NO]; (M) (O2): (M) Initial Rate (M-'s-1) 0.030 0.0055 8.55 x 10-3 0.030 0.0110 1.71 x 10-2 le 0.060 0.0055 3.42 x 10-2 7. Write an expression for the reaction rate law: 1 850 x 11.00 in
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


