Question: Sample Problem 1. a) Write down the Boudouard reaction... b) Calculate the equilibrium ratio: PCO2PCO2 for the Boudouard reaction at 1900K. c) Calculate the composition
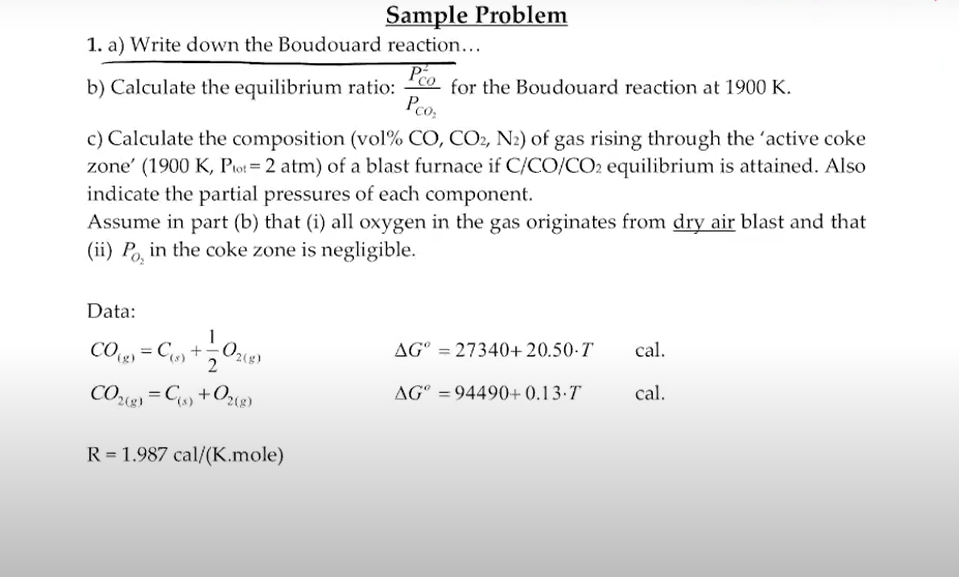
Sample Problem 1. a) Write down the Boudouard reaction... b) Calculate the equilibrium ratio: PCO2PCO2 for the Boudouard reaction at 1900K. c) Calculate the composition (vol\% CO,CO2,N2 ) of gas rising through the 'active coke zone' (1900K,Ptot=2atm) of a blast furnace if C/CO/CO2 equilibrium is attained. Also indicate the partial pressures of each component. Assume in part (b) that (i) all oxygen in the gas originates from dry air blast and that (ii) PO2 in the coke zone is negligible. Data: CO(g)=C(s)+21O2(g)CO2(g)=C(s)+O2(g)Go=27340+20.50TGo=94490+0.13Tcal.cal. R=1.987cal/(K.mole)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


