Question: Sample Problem. An internal combustion engine having a thermal efficiency of 25 percent produces 2500 joules of work. (a) how much is the input heat?
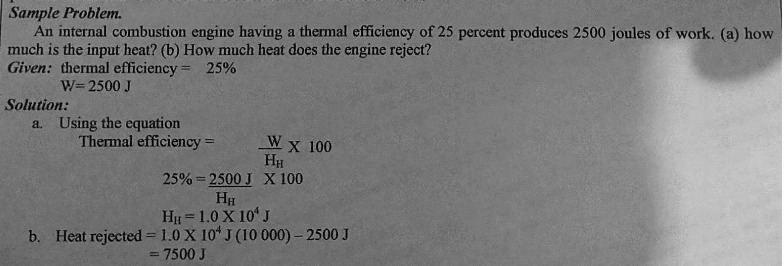
Sample Problem. An internal combustion engine having a thermal efficiency of 25 percent produces 2500 joules of work. (a) how much is the input heat? (b) How much heat does the engine reject? Given: thermal efficiency = 25% W=2500 J Solution: a. Using the equation Thermal efficiency = W X 100 HH 25% = 2500 J X 100 HH HH = 1.0 X 10* J b. Heat rejected = 1.0 X 10# J (10 000) - 2500 J = 7500 J
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


