Question: Save An Question 5 5 points In a bomb calorimeter containing 980 g of water, 0.8221 g of succinic acid is burned. During this reaction
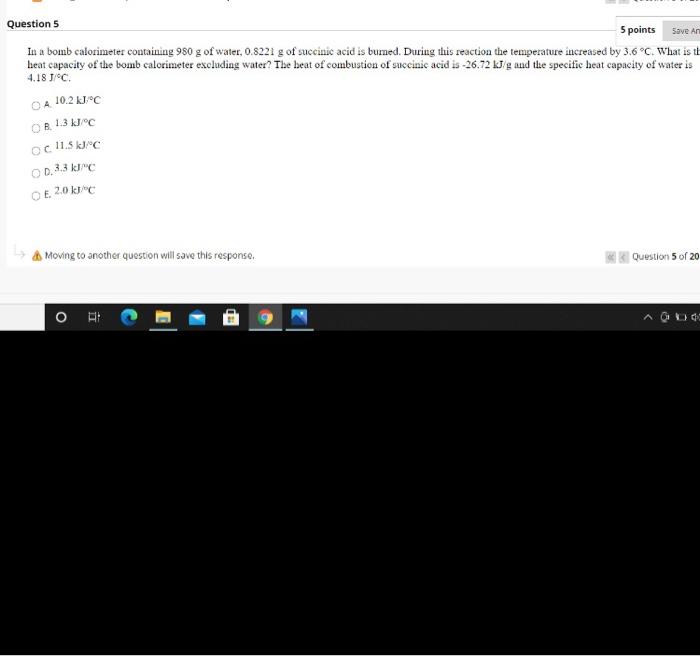
Save An Question 5 5 points In a bomb calorimeter containing 980 g of water, 0.8221 g of succinic acid is burned. During this reaction the temperature increased by 3.6C. What is it heat capacity of the bomb calorimeter excluding water? The beat of combustion of succinic acid is-26.72 kJ/g and the specific heat capacity of water is 4.18 C. 10.2 C OA OR 1.3 k1C OC 11.5 kJ/ OD.3.3klc E. 2.0 kc Moving to another question will save this response. Question 5 of 20 OR A
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


