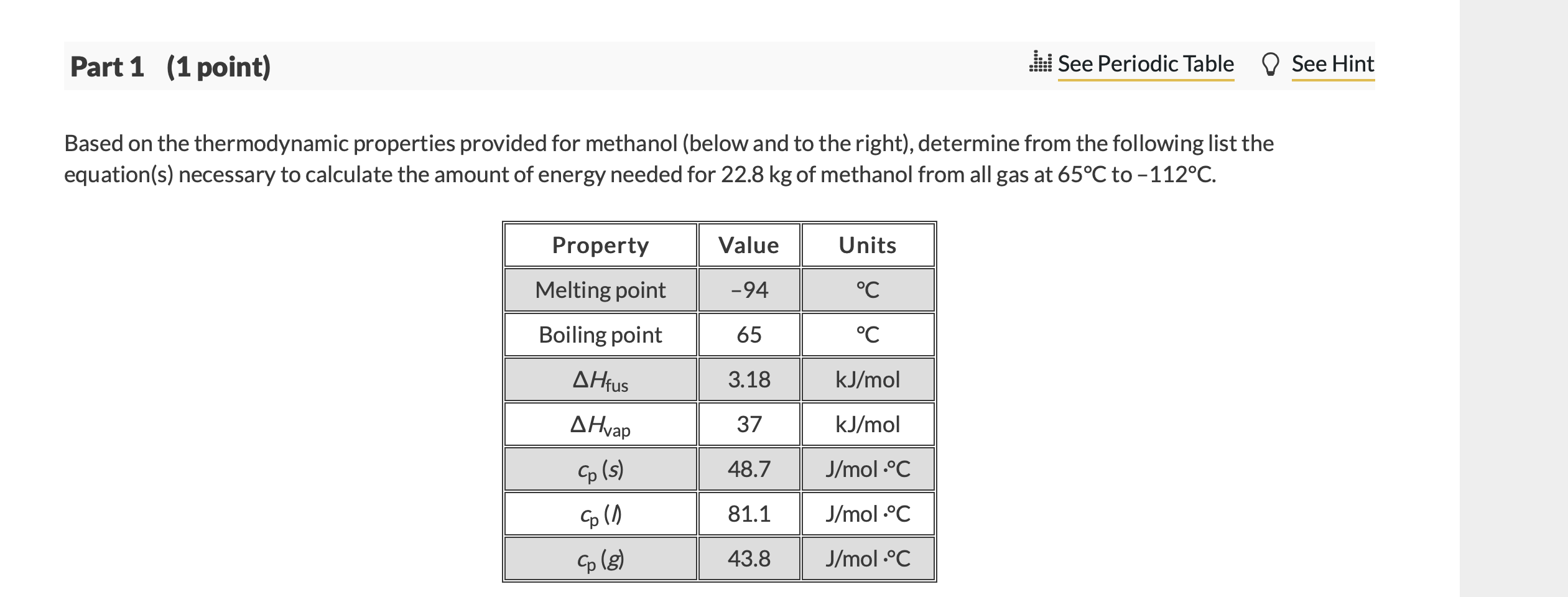
Question: says its wrong Part 1 (1 point) . See Periodic Table D See Hint Based on the thermodynamic properties provided for methanol (below and to
 says its wrong
says its wrong
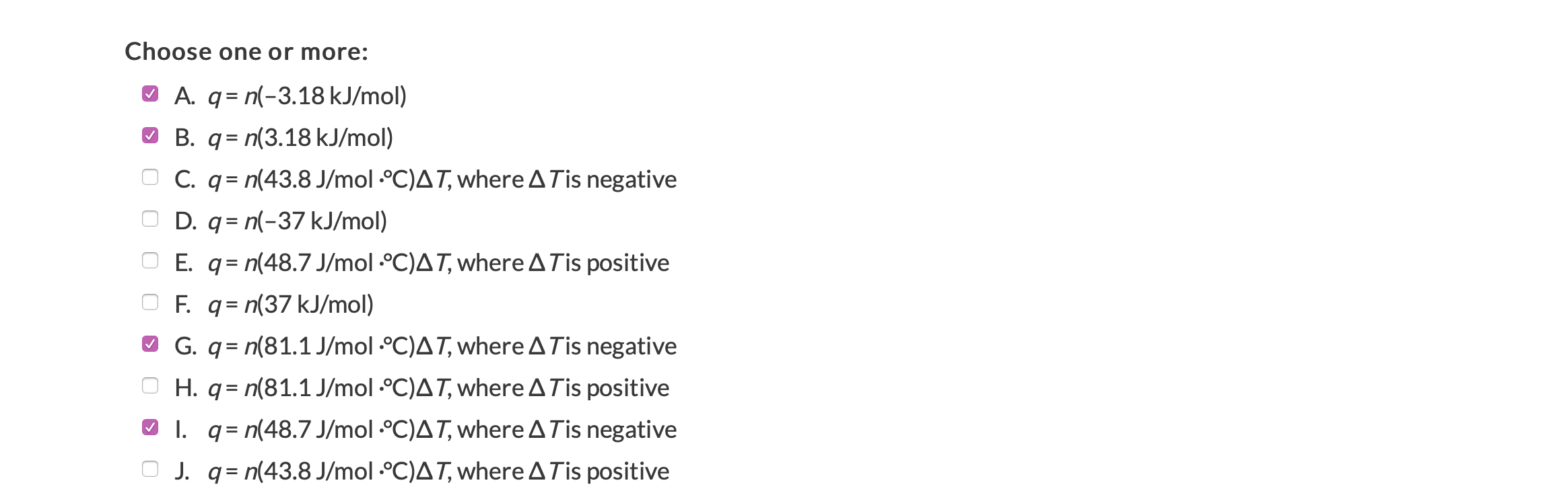
Part 1 (1 point) . See Periodic Table D See Hint Based on the thermodynamic properties provided for methanol (below and to the right), determine from the following list the equation(s) necessary to calculate the amount of energy needed for 22.8 kg of methanol from all gas at 65C to -112C. Property Value Units Melting point -94 C Boiling point 65 C AHfus 3.18 kJ/mol AHvap 37 kJ/mol co(s) 48.7 J/mol C Co (1) 81.1 J/mol C Cp (g) 43.8 J/mol C Choose one or more: A. q= n(-3.18 kJ/mol) B. q= n(3.18 kJ/mol) O C. q= n(43.8 J/mol C)AT, where A Tis negative O D. q= n(-37 kJ/mol) E. q= n(48.7 J/mol C)AT, where A Tis positive F. q= n(37 kJ/mol) G. q = n(81.1 J/mol C)AT, where A Tis negative H. q= n(81.1 J/mol C)AT, where A Tis positive o l. q= n(48.7 J/mol C)AT, where A Tis negative J. q= n(43.8 J/mol C)AT, where A Tis positive
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


