Question: Scan and upload the file. Show all work. Find the pH of 0.183MNO2, a weak base. Kb=2.31011. Use the ICE table method. Verify your answer
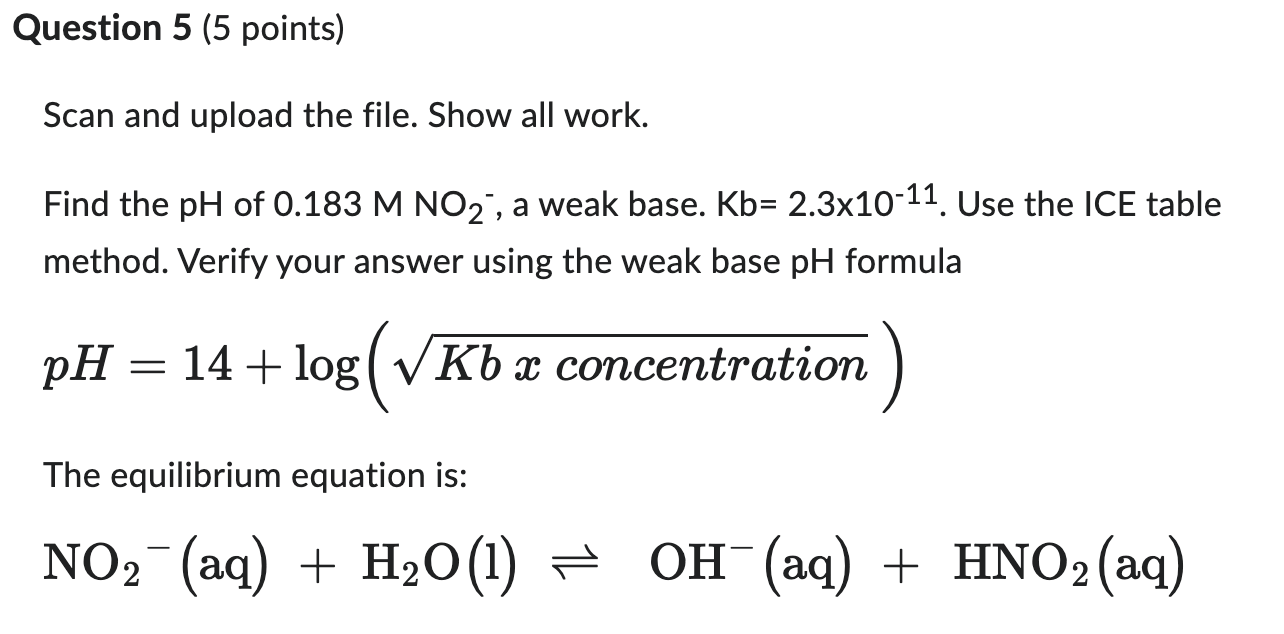
Scan and upload the file. Show all work. Find the pH of 0.183MNO2, a weak base. Kb=2.31011. Use the ICE table method. Verify your answer using the weak base pH formula pH=14+log(Kbxconcentration) The equilibrium equation is: NO2(aq)+H2O(l)OH(aq)+HNO2(aq)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


