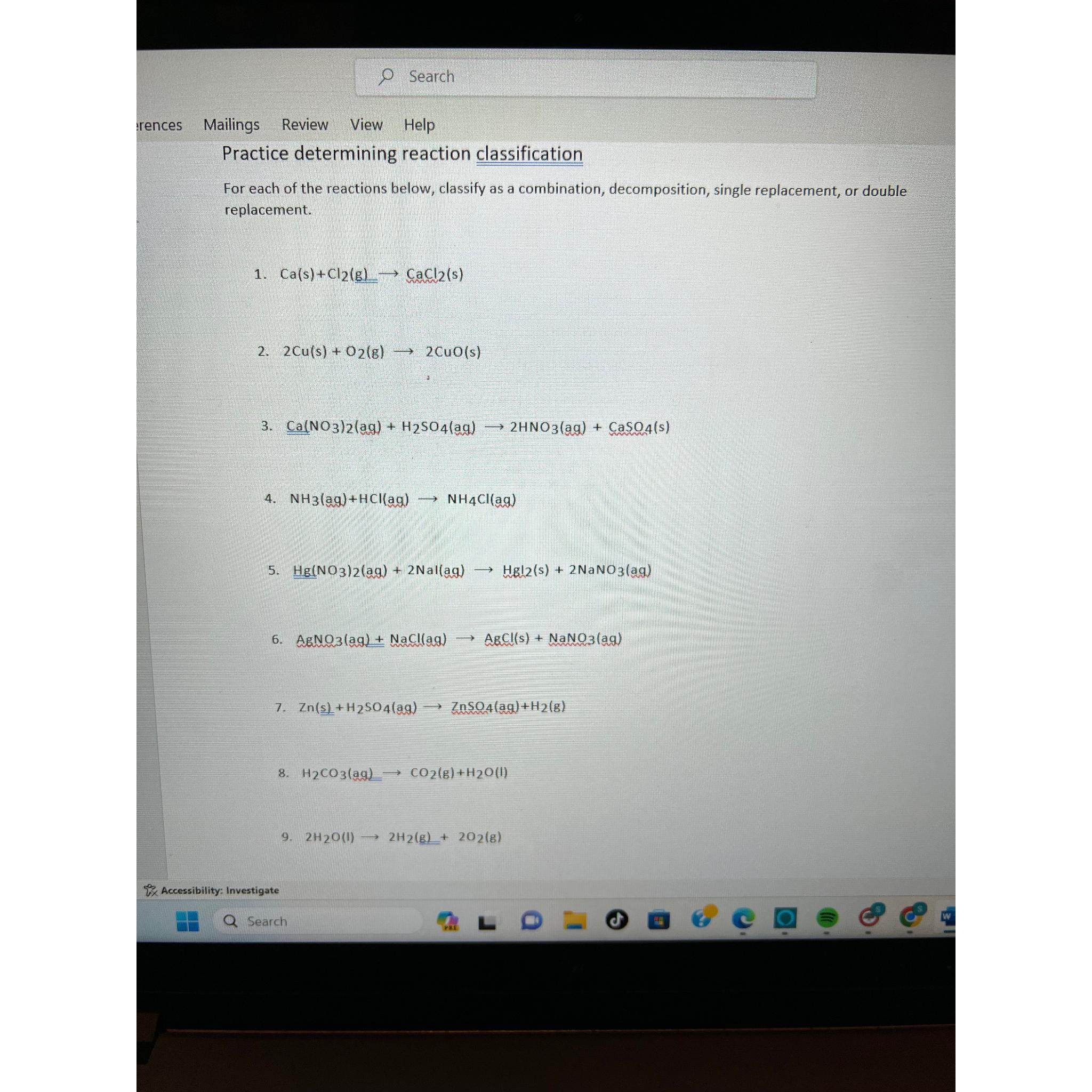
Question: Search rences Mailings Review View Help Practice determining reaction classification For each of the reactions below, classify as a combination, decomposition, single replacement, or double
Search
rences Mailings Review View Help
Practice determining reaction classification
For each of the reactions below, classify as a combination, decomposition, single replacement, or double replacement.
CuO
NaI
NaClAgCl
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


