Question: Second guessing myself again! Please help, I would really appreciate this! For each system listed in the first column of the table below, decide (if
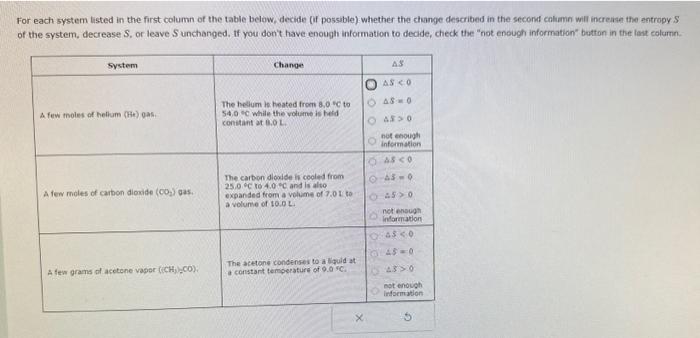
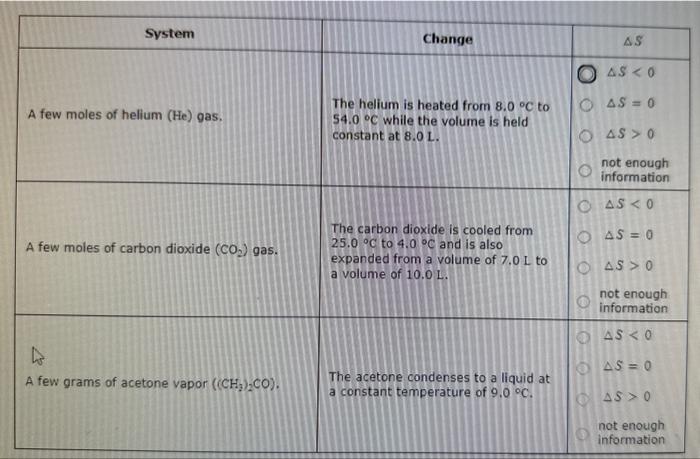

For each system listed in the first column of the table below, decide (if possible) whether the change described in the second caluamn will increise the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the iant eolurn. For each system listed in the first column of the table below. decide (if possible) whether the change described in the second calumn wilf increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to deade, check the "not enough information" button in the last column
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


