Question: Section 13.5 Q and K 1. Mastery - Equilibrium Constant: Ma... 7 pls M 2. Mastery - Reaction Quotient: Cal 1 pts 2reg 3. Mastery
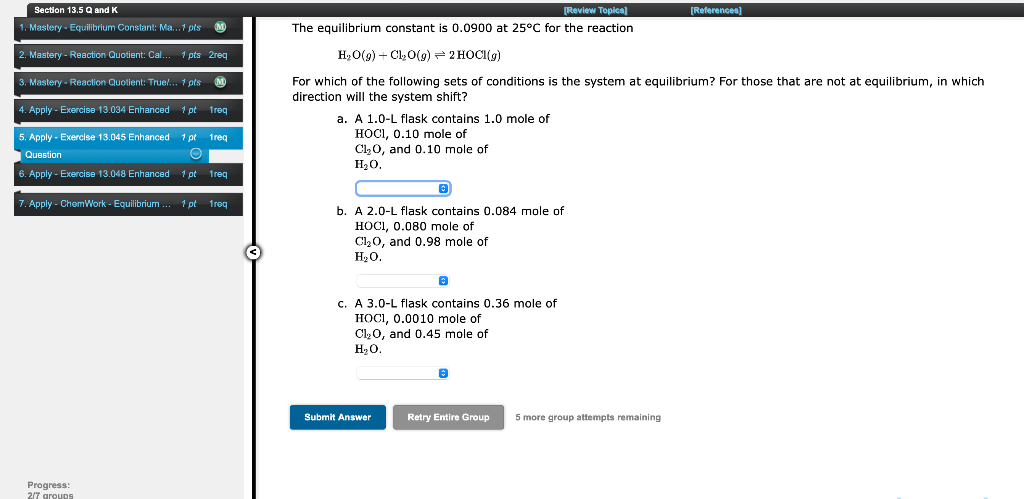
Section 13.5 Q and K 1. Mastery - Equilibrium Constant: Ma... 7 pls M 2. Mastery - Reaction Quotient: Cal 1 pts 2reg 3. Mastery - Reaction Quotient: Truel... 1 pts M [Review Topics] [References) The equilibrium constant is 0.0900 at 25C for the reaction H2O() + C10(9) = 2 HOCI(g) For which of the following sets of conditions is the system at equilibrium? For those that are not at equilibrium, in which direction will the system shift? a. A 1.0-L flask contains 1.0 mole of HOCI, 0.10 mole of C120, and 0.10 mole of H20 4. Apply - Exercise 13.034 Enhanced 1 pt freq 5. Apply - Exercise 13.045 Enhanced 1 pt treg Question 6. Apply - Exercise 13.048 Enhanced 1 pt freq Apply - ChemWork - Equilibrium 1 pt freq b. A 2.0-L flask contains 0.084 mole of HOCI, 0.080 mole of C1,0, and 0.98 mole of HO. C. A 3.0-L flask contains 0.36 mole of HOCI, 0.0010 mole of Cl, O, and 0.45 mole of HO. Submit Answer Retry Entire Group 5 more group attempts remaining Progress: 27 groups
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


