Question: Section B - answer ANY TWO questions from this section B 1 . A stream of air ( 1 0 0 k m o l
Section B answer ANY TWO questions from this section
B A stream of air enriched with needs to be
scrubbed to reduce the concentration. Water has been chosen as
the liquid phase for this process. You may assume the process takes
place at ambient pressure and
a Consider the case where the content of the inlet air is vol
you may assume the rest of air consists of and
and at least of this needs to be removed. What is the
minimum amount of pure water required to achieve this?
marks
b Under the conditions in a how much and are absorbed by
the water stream?
marks
c Water usually contains a small amount of dissolved gases.
Assuming the level in the water being used is in equilibrium
with ambient air ie air containing what is the
minimum flow rate of water required to achieve removal?
marks
Additional information for question B is given on the next page.Data:
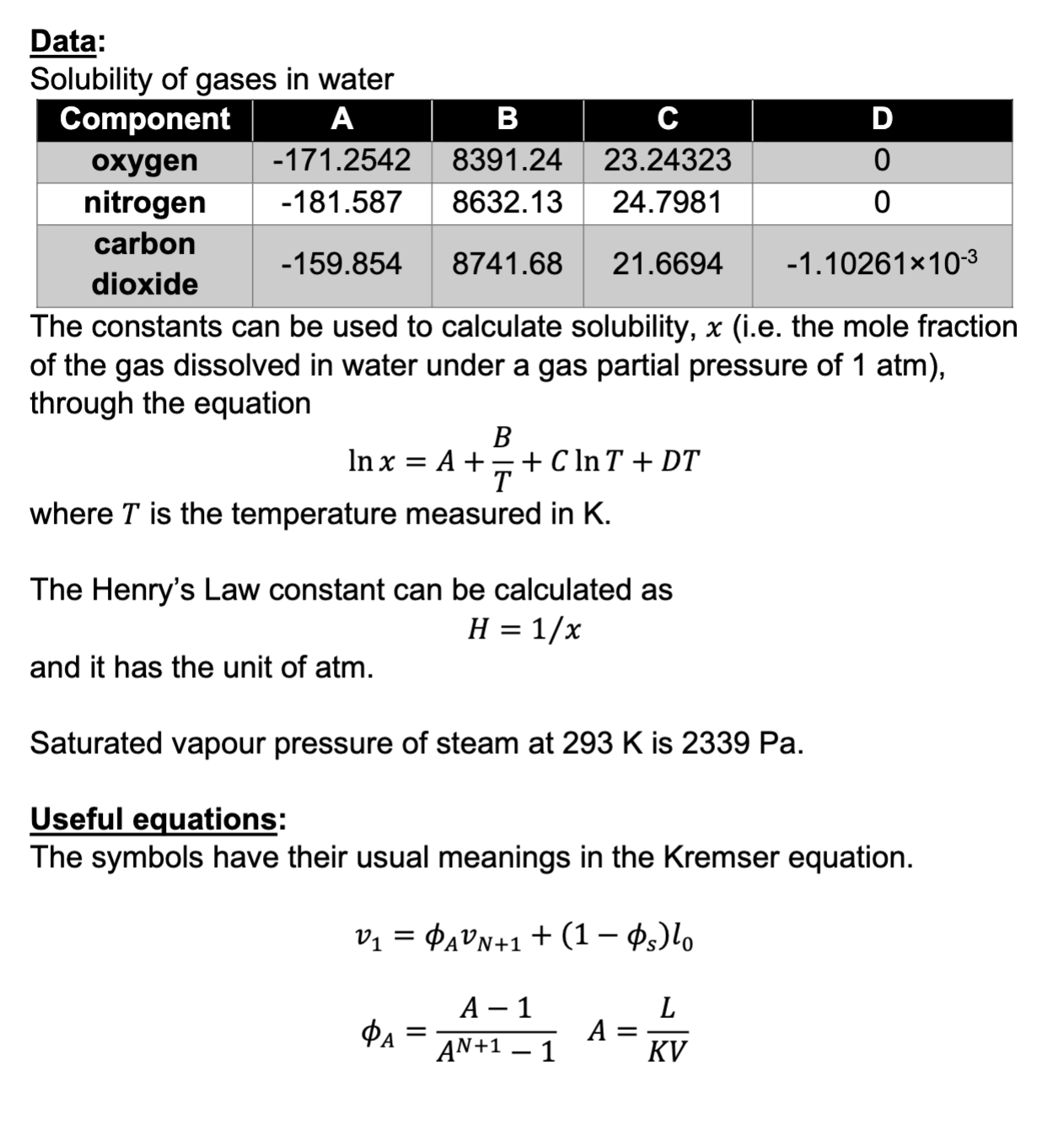
Solubility of gases in water
The constants can be used to calculate solubility, ie the mole fraction
of the gas dissolved in water under a gas partial pressure of atm
through the equation
where is the temperature measured in
The Henry's Law constant can be calculated as
and it has the unit of atm.
Saturated vapour pressure of steam at is
Useful equations:
The symbols have their usual meanings in the Kremser equation.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


