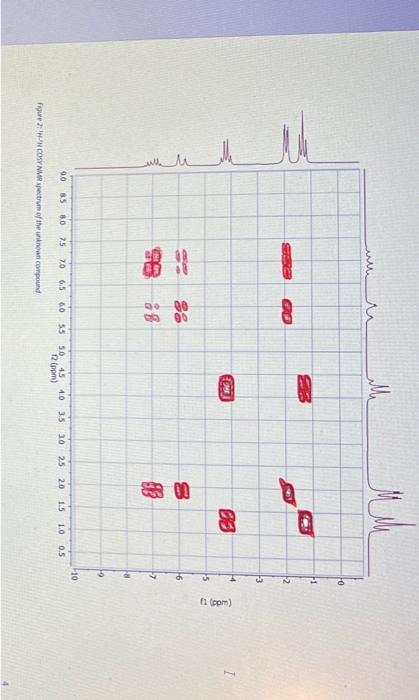
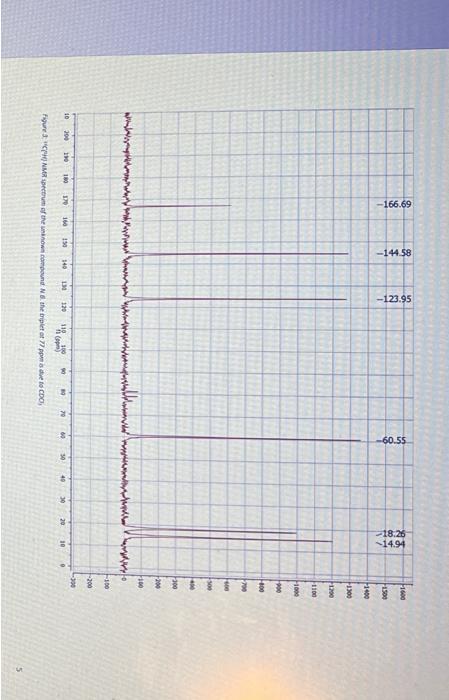
Question: Section B Questions (75 marks) Hint: The unknown compound comprises of C,H and O only. CHN data and required mass, 1H1H COSY, 13C{1H, DEPT-45, DEPT-

![deduce the empirical formula for the unknown compound [3] 2. Using the](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6cc918d6b9_25766f6cc912c207.jpg)
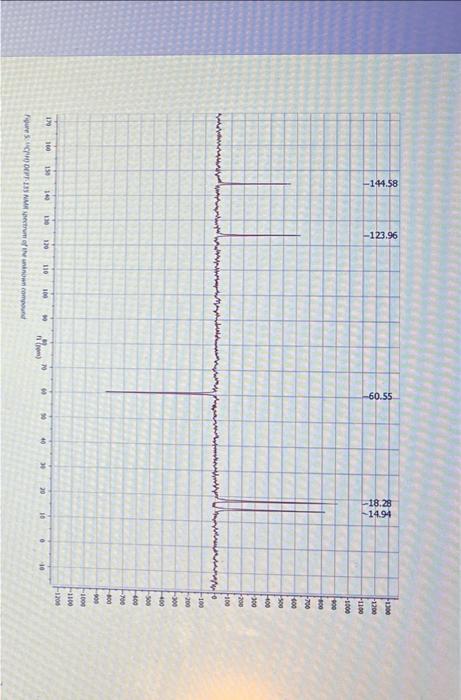
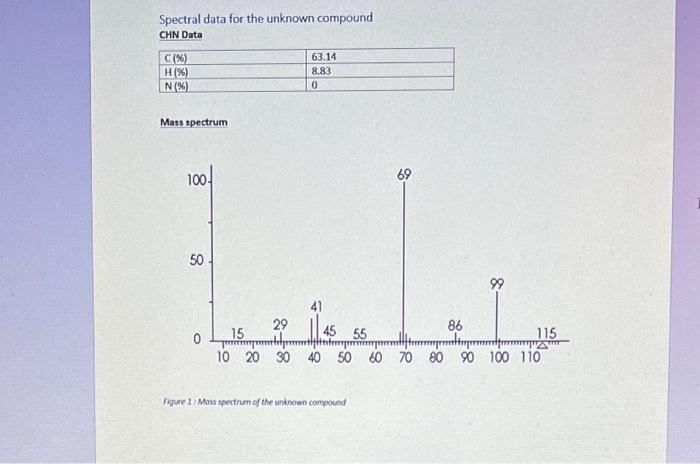
Section B Questions (75 marks) Hint: The unknown compound comprises of C,H and O only. CHN data and required mass, 1H1H COSY, 13C{1H, DEPT-45, DEPT- 135 NMR spectra follow the questions YOU MUST SHOW YOUR WORKING OUT TO ACHIEVE FULL MARKS 1. Using the CHN data given deduce the empirical formula for the unknown compound [3] 2. Using the mass spectra provided, and your empirical formulae, deduce the molecular formula for the unknown compound. Draw out the chemical species that is responsible for the base peak and stipulate why this is the most stable species (you may want to use a diagram here). What is the fragment responsible for the peak at m/z=99 ? 3. Attach to your report a labelled IR spectrum whereby key stretches are identified and assigned. You should also state on the IR spectrum which functional groups can be ruled out AND which are potentially present (use the answers to questions 1 and 2 in this section to help here). 4. Attach to your report your 1H NMR spectrum. To score full marks, your spectrum must be integrated, peaks labelled with chemical shifts (to four decimal places, a total of 27 should be visible), referenced appropriately (the centre of the most ypfield peak should be 1.3ppm or referenced to TMS at 0ppm ), titled appropriately (including your name) and the x-axis labelled as "Chemical shift/ppm". Follow the video on Moodle for further help [5] Spectral data for the unknown compound CHN Data Mass spectrum Figure 1: Mass spertrum of the uninown compound
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


