Question: ) Section il-Select the correct answer (4 pts each) (11) Which of the following does the van der Waals equation correct for? (A) The effect
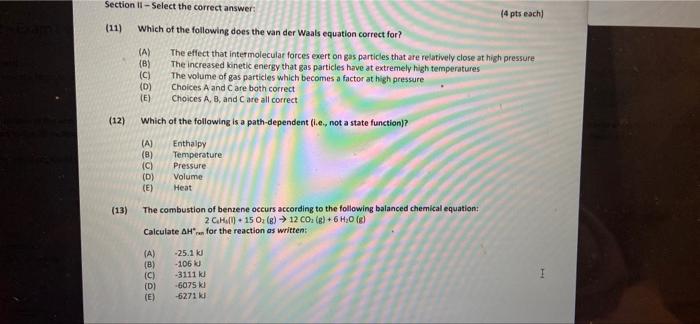
) Section il-Select the correct answer (4 pts each) (11) Which of the following does the van der Waals equation correct for? (A) The effect that intermolecular forces exert on gas particles that are relatively close at high pressure (B) The increased kinetic energy that gas particles have at extremely high temperatures (c) The volume of gas particles which becomes a factor at high pressure (D) Choices A and Care both correct TE) Choices A, B, and Care all correct (12) Which of the following is a path-dependent (le, not a state function)? @ (A) (8) (C) (D) (E) Enthalpy Temperature Pressure Volume Heat (13) The combustion of benzene occurs according to the following balanced chemical equation: 2CH() +15 0(8) 12 CO. (8) + 6H30 () Calculate Arun for the reaction as written: (A) (B) (C) (D) (E) -25.1k -106 -3111 kl -6075 l -62710
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


