Question: Seeding a precipitative softening reactor with calcite can sometimes increase the efficiency of the process. Precipitation on the pre - existing solids requires transport of
Seeding a precipitative softening reactor with calcite
can sometimes increase the efficiency of the process.
Precipitation on the preexisting solids requires transport
of calcium to the particle surface carbonate is
also required, but calcium supply seems to limit the
reaction followed by the precipitation reaction, in
which the ions are incorporated into the solid. A
number of researchers eg Chao and Westerhoff,
have modeled the overall reaction as irreversible and second order with respect to the
calcium concentration rcalcite kCa
In a system containing a bulk Ca concentration
of molL the overall reaction rate normalized
to the calcite surface area is molm
min. The diffusion coefficient for Ca in the solution
is cms the diameter of the seed particles
is mm and the water temperature is C For the
experimental conditions, the mass transfer coefficient
for Ca transport through the liquid boundary layer
around the particles can be estimated based on the
following correlation for mass transfer during flow
around a sphere:
where DrrprL and the other parameters have
their usual meanings. The density of calcite is
gcm
a What is the surfacenormalized rate constant for
the precipitation reaction, in Lm min mol
b What is the mass transfer coefficient ms for
Ca transport through the boundary layer in
this system?
c What is the concentration molL of calcium at
the particlewater interface? What do you infer
about the resistance associated with mass transfer
compared to that associated with the incorporation
step? Briefly explain how you reached
your conclusion.
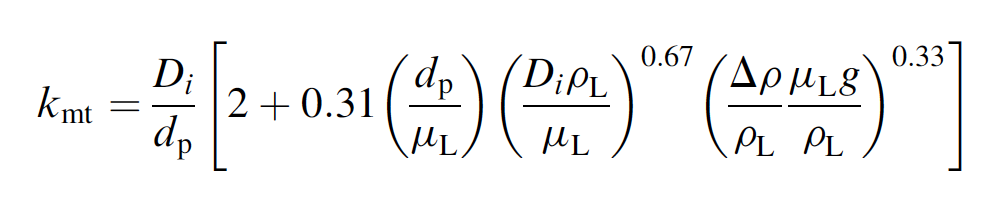
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


