Question: Select any/all of the solutions shown which represent a buffer system after mixing. 100.0mL of 0.10MHNO3(aq)+100.0mL of 0.10MNaNO3(aq) 100.0mL of 0.10MH2CO3(aq)+100.0mL of 0.10MNaHCO3(aq) 100.0mL of
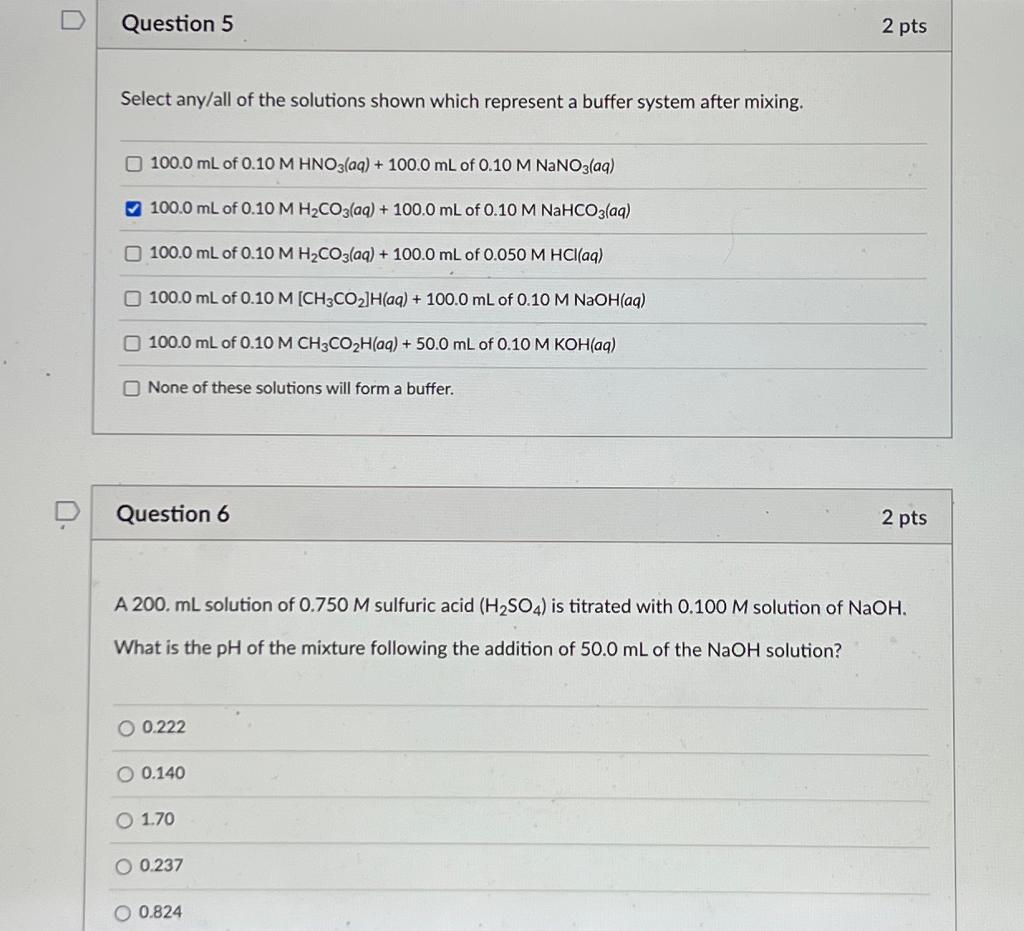
Select any/all of the solutions shown which represent a buffer system after mixing. 100.0mL of 0.10MHNO3(aq)+100.0mL of 0.10MNaNO3(aq) 100.0mL of 0.10MH2CO3(aq)+100.0mL of 0.10MNaHCO3(aq) 100.0mL of 0.10MH2CO3(aq)+100.0mL of 0.050MHCl (aq) 100.0mL of 0.10M[CH3CO2]H (aq) +100.0mL of 0.10MNaOH( aq ) 100.0mL of 0.10MCH3CO2H( aq )+50.0mL of 0.10MKOH( aa) None of these solutions will form a buffer. Question 6 2 pts A 200. mL solution of 0.750M sulfuric acid (H2SO4) is titrated with 0.100M solution of NaOH. What is the pH of the mixture following the addition of 50.0mL of the NaOH solution? 0.222 0.140 1.70 0.237 0.824
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


