Question: Select one: a. Ts b. Og+ c. Mc d. Nh Consider a reaction that has H=365kmol1 and S=120JK1mol1 Which of the following statements about the
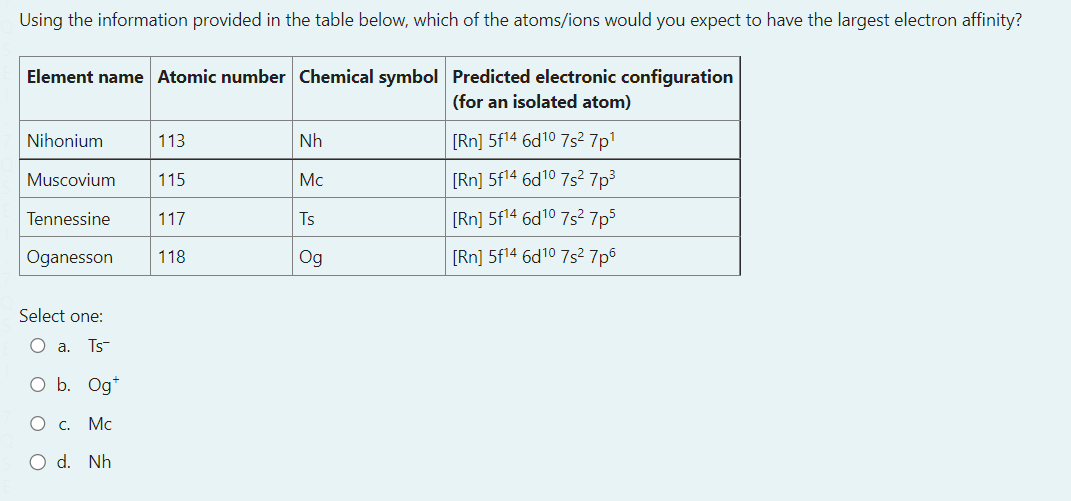

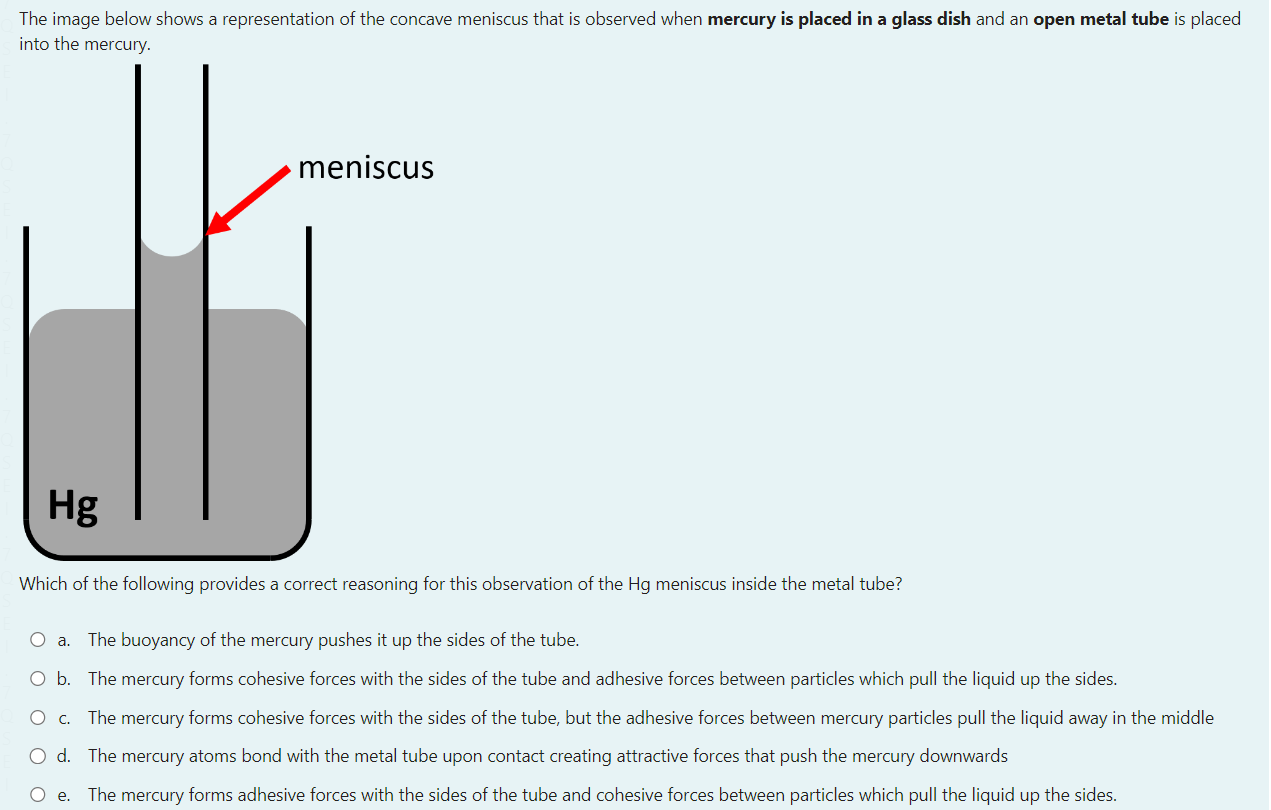
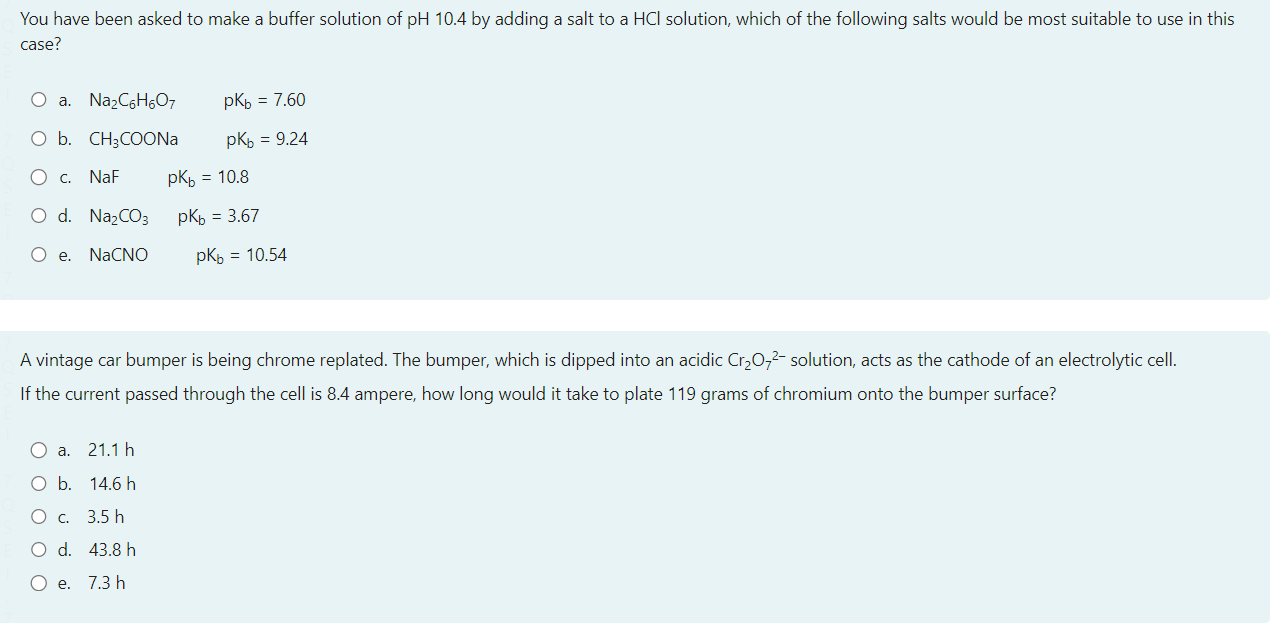
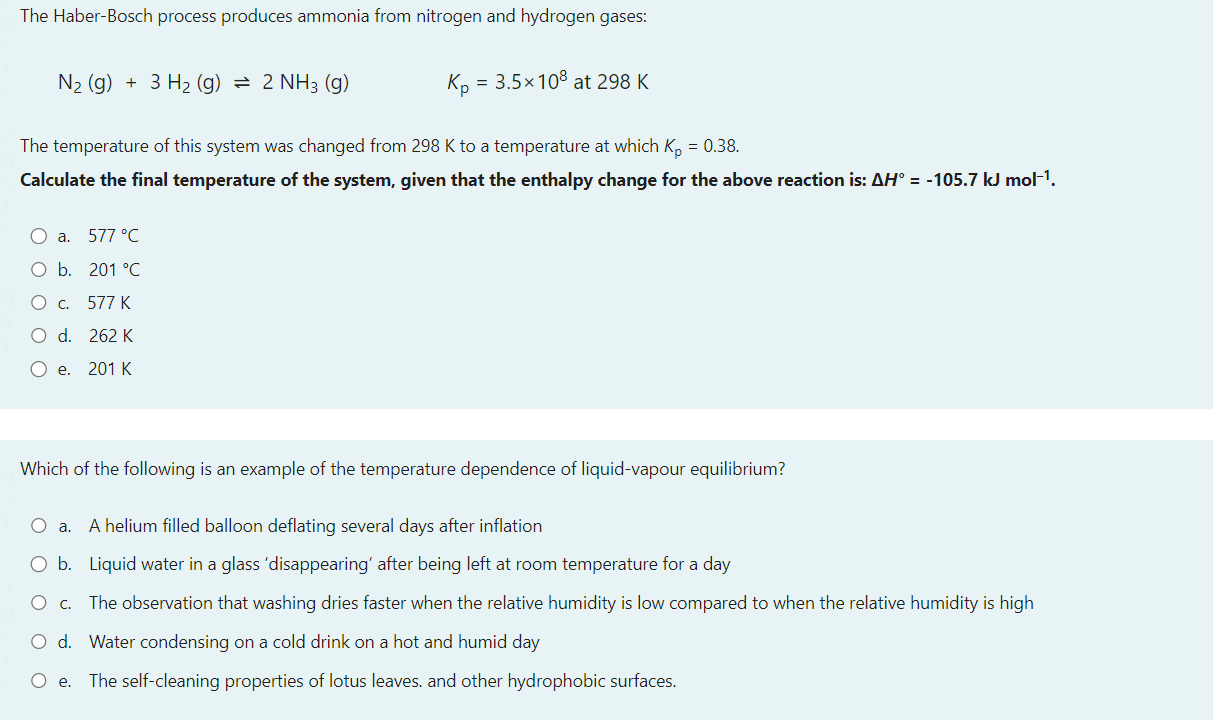
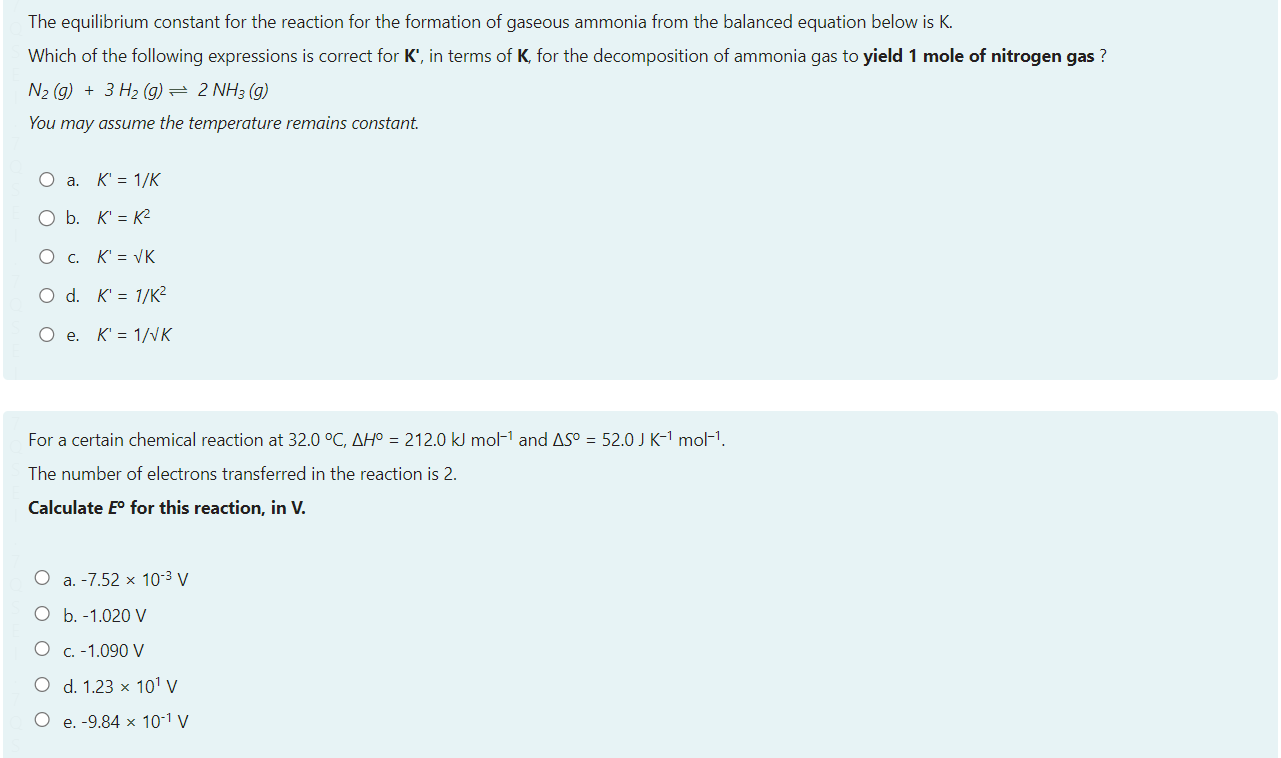
Select one: a. Ts b. Og+ c. Mc d. Nh Consider a reaction that has H=365kmol1 and S=120JK1mol1 Which of the following statements about the spontaneity of the reaction applies to this reaction? a. The reaction will never be spontaneous regardless of temperature b. The reaction will be non-spontaneous at low temperatures, and spontaneous at high-temperatures. c. You cannot determine if the reaction is spontaneous from the information provided. d. The reaction will be spontaneous at low temperatures, and non-spontaneous at high-temperatures. e. The reaction will always be spontaneous regardless of temperature The image below shows a representation of the concave meniscus that is observed when mercury is placed in a glass dish and an open metal tube is placed into the mercury. Which of the following provides a correct reasoning for this observation of the Hg meniscus inside the metal tube? a. The buoyancy of the mercury pushes it up the sides of the tube. b. The mercury forms cohesive forces with the sides of the tube and adhesive forces between particles which pull the liquid up the sides. c. The mercury forms cohesive forces with the sides of the tube, but the adhesive forces between mercury particles pull the liquid away in the midde d. The mercury atoms bond with the metal tube upon contact creating attractive forces that push the mercury downwards e. The mercury forms adhesive forces with the sides of the tube and cohesive forces between particles which pull the liquid up the sides. You have been asked to make a buffer solution of pH10.4 by adding a salt to a HCl solution, which of the following salts would be most suitable to use in this case? a. Na2C6H6O7pKb=7.60 b. CH3COONapKb=9.24 c. NaFpKb=10.8 d. Na2CO3pKb=3.67 e. NaCNOpKb=10.54 A vintage car bumper is being chrome replated. The bumper, which is dipped into an acidic Cr2O72 solution, acts as the cathode of an electrolytic cell. If the current passed through the cell is 8.4 ampere, how long would it take to plate 119 grams of chromium onto the bumper surface? a. 21.1h b. 14.6h c. 3.5h d. 43.8h e. 7.3h N2(g)+3H2(g)2NH3(g)Kp=3.5108at298K The temperature of this system was changed from 298K to a temperature at which Kp=0.38. Calculate the final temperature of the system, given that the enthalpy change for the above reaction is: H=105.7kJmol1. a. 577C b. 201C c. 577K d. 262K e. 201K Which of the following is an example of the temperature dependence of liquid-vapour equilibrium? a. A helium filled balloon deflating several days after inflation b. Liquid water in a glass 'disappearing' after being left at room temperature for a day c. The observation that washing dries faster when the relative humidity is low compared to when the relative humidity is high d. Water condensing on a cold drink on a hot and humid day e. The self-cleaning properties of lotus leaves. and other hydrophobic surfaces. The equilibrium constant for the reaction for the formation of gaseous ammonia from the balanced equation below is K. Which of the following expressions is correct for K, in terms of K, for the decomposition of ammonia gas to yield 1 mole of nitrogen gas ? N2(g)+3H2(g)2NH3(g) You may assume the temperature remains constant. a. K=1/K b. K=K2 c. K=K d. K=1/K2 e. K=1/K For a certain chemical reaction at 32.0C,H=212.0kJmol1 and S=52.0JK1mol1. The number of electrons transferred in the reaction is 2. Calculate E for this reaction, in V. a. 7.52103V b. 1.020V c. 1.090V d. 1.23101V e. 9.84101V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


