Question: Select the compound below that you would expect to have the higher boiling point. Select the best explanation for your choice in part (a). The
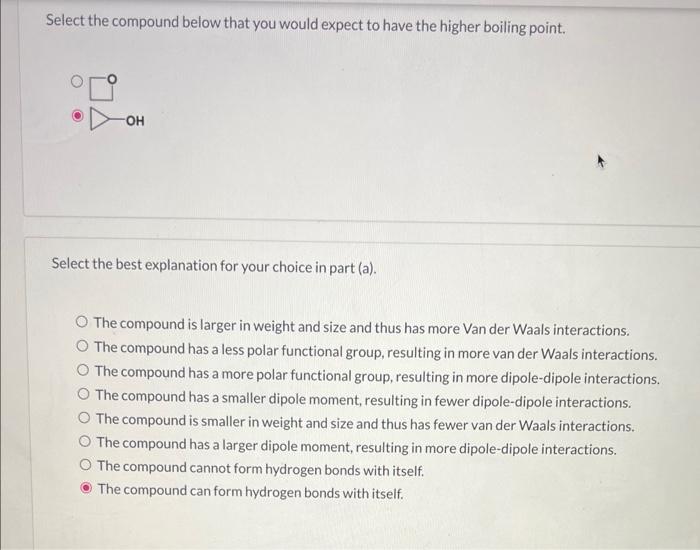
Select the compound below that you would expect to have the higher boiling point. Select the best explanation for your choice in part (a). The compound is larger in weight and size and thus has more Van der Waals interactions. The compound has a less polar functional group, resulting in more van der Waals interactions. The compound has a more polar functional group, resulting in more dipole-dipole interactions. The compound has a smaller dipole moment, resulting in fewer dipole-dipole interactions. The compound is smaller in weight and size and thus has fewer van der Waals interactions. The compound has a larger dipole moment, resulting in more dipole-dipole interactions. The compound cannot form hydrogen bonds with itself. The compound can form hydrogen bonds with itself
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


